Is the combustion of ethane, C 2 H 6 , product-favored at equilibrium at 25C? C 2
Question:
Is the combustion of ethane, C2H6, product-favored at equilibrium at 25°C?
C2H6(g) + 7⁄2 O2(g) → 2 CO2(g) + 3 H2O(g)
Answer the question by calculating the value of ΔS°(universe) at 298 K, using values of ΔfH° and S° in Appendix L. Does the answer agree with your preconceived idea of this reaction?
Data given in Appendix L
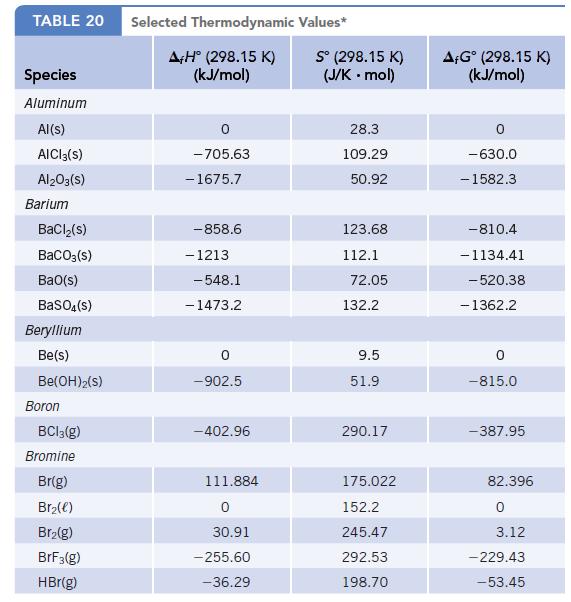
Transcribed Image Text:
TABLE 20 Species Aluminum Al(s) AICI 3(S) Al2O3(S) Barium BaCl₂(s) BaCO3(s) BaO(s) BaSO4(s) Beryllium Be(s) Be(OH)2(S) Boron BC13(g) Bromine Br(g) Br₂(e) Br₂(g) BrF3(g) HBr(g) Selected Thermodynamic A+H° (298.15 K) (kJ/mol) -705.63 - 1675.7 -858.6 - 1213 -548.1 -1473.2 0 -902.5 -402.96 111.884 0 30.91 -255.60 -36.29 Values* Sº (298.15 K) (J/K .mol) 28.3 109.29 50.92 123.68 112.1 72.05 132.2 9.5 51.9 290.17 175.022 152.2 245.47 292.53 198.70 AFGᵒ (298.15 K) (kJ/mol) -630.0 - 1582.3 -810.4 -1134.41 -520.38 - 1362.2 0 -815.0 -387.95 82.396 0 3.12 -229.43 -53.45
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
CH72028 2CO2g 3HO 8 AH for CH 8385 KJmol AH for O20...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Draw a free body diagram of an individual performing calfraises, rising onto the balls of their feet and then lowering theirheels. Draw a free body diagram of an individual performing overheadtriceps...
-
The production of maleic anhydride by the air oxidation of benzene was studied using a vanadium pentoxide catalyst [Chem Eng Sci, 43, 1051 (1988)]. The reactions that occur are C6H6 + 9/ 2 O2 C4H2O3...
-
Kim was single on December 31,2021. Her husband, Lee, passed away on march 20, 2018, and she has not remarried. Rebecca and Doug have always filed married filing jointly in previous tax years. Kim...
-
Verde Company produces wheels for bicycles. During the year, 657,000 wheels were produced. The actual labor used was 368,000 hours at $9.50 per hour. Verde has the following labor standards: 1)...
-
Why are there more accountants per head of population in New Zealand than in France?
-
An employee worked as a drill and machine operator at a furniture manufacturer. Employees were subject to dismissal when they accumulated seven points against them for violations based on conduct....
-
On presenting your manager with the differential analysis of two possible uses for a piece of land that cost the company $1.5 million your manager believes you have made a major error as you omitted...
-
Agatha is planning to start a new business venture and must decide whether to operate as a sole proprietorship or incorporate. She projects that the business will generate annual cash flow and...
-
Quirk Corporation issued a 100% stock dividend of its $10 par value common stock. What amount of retained earnings will be capitalized for the additional shares issued?
-
When vapors from hydrochloric acid and aqueous ammonia come in contact, they react, producing a white cloud of solid NH 4 Cl (Figure 18.9). HCl(g) + NH 3 (g) NH 4 Cl(s) Defining the reactants and...
-
Hydrogenation, the addition of hydrogen to an organic compound, is an industrially important reaction. Calculate r H, r S, and r G for the hydrogenation of octene, C 8 H 16 , to give octane, C 8 H...
-
Fancy Free Social Club was established with the main aim of promoting jogging, cycling and indoor exercise. Their cash flow for the year ended 31 May 20x2 is summarised below: Their assets and...
-
Brett Arends, a columnist for the Wall Street Journal, argues: Today you should probably view [financial firms selling investments] the way you view someone selling a used car. How should you view...
-
Suppose that you have $1,000 to invest in the bond market on January 1, 2014. You could buy a one-year bond with an interest rate of 4%, a two-year bond with an interest rate of 5%, a three-year bond...
-
An article in the Economist magazine on crowd-funding argued: Start-ups are especially needy now, since many banks are loth to lend even to well-established companies. a. Why might banks be reluctant...
-
An article in the Wall Street Journal notes that investors tend to view [preferred stock] more like bonds than like [common] stock. a. In what sense is preferred stock more like bonds than like...
-
An article in the Economist magazine observes: Insurance companies often suspect the only people who buy insurance are the ones most likely to collect. a. What do economists call the problem being...
-
Refer to the information presented in M6-14. Suppose that each products sales price increases by 10 percent. Sales mix remains the same and total fixed costs are $230,000. Calculate the new...
-
In July 2013, cnet.com listed the battery life (in hours) and luminous intensity (i. e., screen brightness, in cd/m2) for a sample of tablet computers. We want to know if screen brightness is...
-
If the probability density of finding the electron in the 1s orbital in the H atom has its maximum value for r = 0, does this mean that the proton and electron are located at the same point in space?
-
Just as for the finite depth box, wave functions for which E-V 0 is small (3s) penetrate further into the barrier than wave functions for which E-V 0 is large (1s). The 2s wave function is...
-
What are the units of the H atom total energy eigenfunctions? Why is a 0 3/2 R(r) graphed in Figure 20.6 rather than R(r)?
-
How can we integrate advanced automation techniques to augment efficiency and mitigate resource wastage? Explain with Example
-
The owner invested $15,000 in a business. Dr. Cash $15,000 Cr. Owner's Capital $15,000 Note - When the Owner contributes any kind of capital, it will always impact the Owner's Capital account. From...
-
x+12 The function f(x)= is one-to-one. For the function, x-4 a. Find an equation for f (x), the inverse function. b. Verify that your equation is correct by showing that f((x)) =x and f(f(x)) = x.

Study smarter with the SolutionInn App


