When vapors from hydrochloric acid and aqueous ammonia come in contact, they react, producing a white cloud
Question:
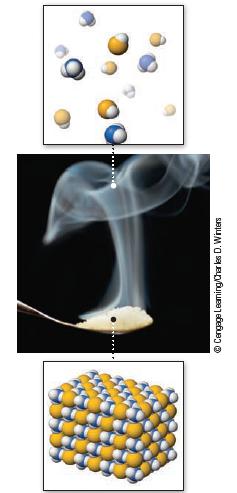
When vapors from hydrochloric acid and aqueous ammonia come in contact, they react, producing a white “cloud” of solid NH4Cl (Figure 18.9).
HCl(g) + NH3(g) ⇄ NH4Cl(s)
Defining the reactants and products as the system under study:
(a) Predict whether ΔS°(system), ΔS°(surroundings), ΔS°(universe), Δr H°, and ΔrG° (at 298 K) are greater than zero, equal to zero, or less than zero; and explain your prediction. Verify your predictions by calculating values for each of these quantities.
(b) Calculate the value of Kp for this reaction at 298 K.
Data given in figure 18.9
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





