Silver ions have long been known to have biocidal properties. The increasing use of silver for this
Question:
Silver ions have long been known to have biocidal properties. The increasing use of silver for this purpose has led to greater silver concentrations in waste water, and there has been some concern about it. However, recent studies found that the silver was largely put back into the environment as Ag2S. The solubility of this sulfide is 1.4 × 10−4 g/L.
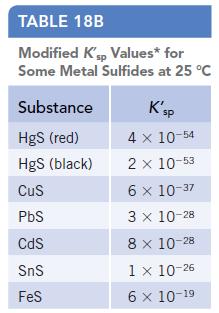
(a) Estimate the K′sp for Ag2S. (See Table 18b in Appendix J.)
(b) How does the solubility of Ag2S change as the pH decreases?
Data given in Table 18b
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





