Sulfuric acid is listed in a catalog with a concentration of 9598%. A bottle of the acid
Question:
Sulfuric acid is listed in a catalog with a concentration of 95–98%. A bottle of the acid in the stockroom states that 1.00 L has a mass of 1.84 kg. To determine the concentration of sulfuric acid in the stockroom bottle, a student dilutes 5.00 mL to 500. mL. She then takes four 10.00-mL samples and titrates each with standardized sodium hydroxide (c = 0.1760 M).
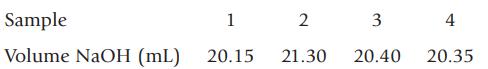
(a) What is the average concentration of the diluted sulfuric acid sample?
(b) What is the mass percent of H2SO4 in the original bottle of the acid?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





