Thallium has two stable isotopes, 203 Tl and 205 Tl. Knowing that the atomic weight of thallium
Question:
Thallium has two stable isotopes, 203Tl and 205Tl. Knowing that the atomic weight of thallium is 204.4, which isotope is the more abundant of the two?

Transcribed Image Text:
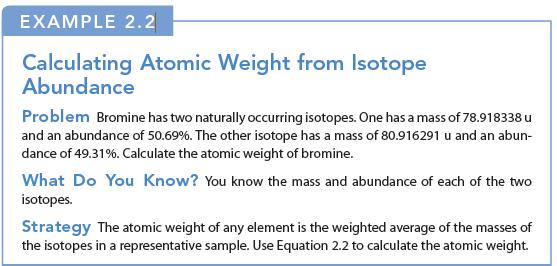
EXAMPLE 2.2 Calculating Atomic Weight from Isotope Abundance Problem Bromine has two naturally occurring isotopes. One has a mass of 78.918338 u and an abundance of 50.69%. The other isotope has a mass of 80.916291 u and an abun- dance of 49.31%. Calculate the atomic weight of bromine. What Do You Know? You know the mass and abundance of each of the two isotopes. Strategy The atomic weight of any element is the weighted average of the masses of the isotopes in a representative sample. Use Equation 2.2 to calculate the atomic weight.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
Thallium has two stable isotopes 20 TI and 205T1 K...View the full answer

Answered By

Saud Ur Rehman
Evaluating manufacturing processes by designing and conducting research programs; applying knowledge of product design, fabrication, assembly, tooling, and materials; conferring with equipment vendors; soliciting observations from operators. Developing manufacturing processes by studying product requirements; researching, designing, modifying, and testing manufacturing methods and equipment; conferring with equipment vendors. Keeping equipment operational by coordinating maintenance and repair services; following manufacturer's instructions and established procedures; requesting special service.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Walton Modems, Incorporated makes modem cards that are used in notebook computers. The company completed the following transactions during year 1. All purchases and sales were made with cash. 1....
-
The density of potassium, which has the BCC structure, is 0.855 g/cm3. The atomic weight of potassium is 39.09 g/mol. Calculate (a) The lattice parameter; and (b) The atomic radius of potassium.
-
The density of thorium, which has the FCC structure and one atom per lattice point, is 11.72 g/cm3. The atomic weight of thorium is 232 g/mol. Calculate (a) The lattice parameter; and (b) The atomic...
-
During the software design phase, software engineers define details about the product construction, behavior, components, and interfaces. Explain how you can use the Unified Modeling Language (UML)...
-
Now you will work on your own to finish up the remaining financial analysis tasks. Again, you can prepare spreadsheet templates, or navigate to the Forms Library (see above). A. Perform ROI and NPV...
-
A firm based in California wants to export a shipload of finished lumber to the Philippines. The would-be importer cannot get sufficient credit from domestic sources to pay for shipment but insists...
-
As discussed in Example 6.7, the following stream at \(100^{\circ} \mathrm{F}\) and 484 psia is to be separated by two distillation columns into the Products \(1-3\) in the following table. Two...
-
Jerry never imagined hed be sitting there in Washington being grilled mercilessly by a panel of congressmen. But a young government auditor picked up on his scheme last year. His company produced...
-
Examine the role of process intensification in developing sustainable and environmentally friendly processes, considering the potential for using renewable feedstocks, reducing waste and emissions,...
-
Cobalt has three radioactive isotopes used in medical studies. Atoms of these isotopes have 30, 31, and 33 neutrons, respectively. Give the complete symbol for each of these isotopes
-
What is the mass of one 16 O atom, in grams? (The mass of an 16 O atom is 15.995 u.)
-
A partial payment is made on the date(s) indicated. Use the United States rule to determine the balance due on the note at the date of maturity. The Effective Date is the date the note was written....
-
What is an example of Requisite Variety that is brought about by Globalization issues and how organizations might address such issues.
-
In the context of cellular respiration, what molecular mechanisms regulate the synthesis and utilization of ATP, the primary energy currency of cells, and how do respiratory substrates like glucose...
-
Factor each polynomial Completely. 2 14z-15z+4 2 2 x - 17x+12 2 4w-14 w (4) 4+2 +12++9
-
(Determining the outstanding balance of a loan) Ten years ago you took out a $300,000, 20-year mortgage with an annual interest rate of 11 percent and monthly payments of $3,096.57. What is the...
-
Actuarial Science An insurer is reviewing claims for a certain line of insurance from Accident year 2 0 2 3 . The earned premiums in 2 0 2 3 were $ 7 . 7 million. The base premium in 2 0 2 3 was $ 1...
-
You have to pick between three mutually exclusive projects with the following cash flows to the firm: The cost of capital is 12%. a. Which project would you pick using the NPV rule? b. Which project...
-
Find the inverse, if it exists, for the matrix. -1
-
Explain why attractive interactions between molecules in gas make the pressure less than that predicted by the ideal gas equation of state.
-
Draw resonance structures for each of the following radicals: (a) (b) (c) (d)
-
Calculate the pressure exerted by benzene for a molar volume of 2.00 L at 595 K using the RedlichKwong equation of state: The RedlichKwong parameters a and b for benzene are 452.0 bar dm 6 mol 2 K...
-
Q) What is spring Framework? How many modules are there in spring framework? Q) Explain spring MVC architecture? What is Spring Boot? Q) In how many ways we can create a spring boot project?...
-
The information for Bland Media Company at the end of the fiscal year, June 30, 20X8 are listed in this spreadsheet . Additional information: Capital were $145000 at May 1, 20X7, additional capital...
-
The information for Bland Media Company at the end of the fiscal year, June 30, 20X8 are listed in this spreadsheet . Additional information: Capital were $145000 at May 1, 20X7, additional capital...

Study smarter with the SolutionInn App


