The equation for the reaction of aluminum and bromine is If you use 6.0 10 23
Question:
The equation for the reaction of aluminum and bromine is ![]() If you use 6.0 × 1023 molecules of Br2 in a reaction how many atoms of Al will be consumed?
If you use 6.0 × 1023 molecules of Br2 in a reaction how many atoms of Al will be consumed?
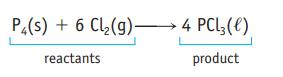
Transcribed Image Text:
2 Al(s) + 3 Br₂(l) → Al₂Bro(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 42% (7 reviews)
To determine how many atoms of aluminum Al will be consumed in the reaction of 2 Als 3 Br2l 2 AlBr3s ...View the full answer

Answered By

AJIN KURIAKOSE
I HAVE ELECTRONICS ENGINEERING DEGREE..AND MY AREA OF INTEREST IS MATHEMATICS,CONTROL SYSTEM,NETWORK,DIGITAL
4.70+
21+ Reviews
32+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Terence purchased a whole life policy in 1977, which he later transferred to his wife Gertrude in 1989. What group would Gertrude's policy fall under for tax purposes? a) G1 b) G2 c) G3 d) G4
-
The equilibrium-constant expression for a reaction is What is the equilibrium-constant expression when the equation for this reaction is halved and then reversed? NO2O IN20512
-
The equilibrium-constant expression for a reaction is What is the equilibrium-constant expression when the equation for this reaction is halved and then reversed? [NH,110,1 K. [NO]'[H,O1
-
The annual consumption of beef per person was about 64 7 lb in 2000 and about 60 3 lb in 2007 Assume B(t), the annual beef consumption t years after 2000, is decreasing according to the exponential...
-
A small country can import a good at a world price of 10 per unit. The domestic supply curve of the good is S = 20 + 10P The demand curve is D = 400 5P In addition, each unit of production yields a...
-
What is the Criminal Investigation Division of the IRS?
-
Consider a Poisson regression. Let \(e_{i}=y_{i}-\widehat{\mu}_{i}\) denote the \(i\) th ordinary residual. Assume that an intercept is used in the model so that one of the explanatory variables...
-
Hammersmith Homes is considering four possible housing development projects, each requiring an initial investment of $5,000,000. The cash inflows from each of the projects follow: a. Compute the net...
-
Explain each as points from the the case "Fruitzone India Limited (B) - Designing the Research Questionnaire". Discussion Points .....but taste is not a TOM attribute of juices...." Comment on this...
-
Oxidation of 1.00 g of carbon monoxide, CO, produces 1.57 g of carbon dioxide, CO 2 . How many grams of oxygen were required in this reaction? P4(s) + 6 Cl(g) 4 PCL3 (1) reactants product
-
Which of the following compounds has the highest mass percent of chlorine? (a) BCl 3 (b) AsCl 3 (c) GaCl 3 (d) AlCl 3 (e) PCl 3
-
What is the winner's curse? Why can the winner's curse arise in a common-values auction but not in a private-values auction?
-
10.27 Find Hamilton's canonical equations for (a) A simple pendulum (b) A simple Atwood machine (c) A particle sliding down a smooth inclined plane
-
A car is moving in a circle at a constant speed of 45 m/s. Its radial acceleration is 60 m/s2. Fill in the data table below. t (s) V (m/s) a (m/s) 0 1 2 3 4
-
Q = mc AT An aluminum metal is heated in boiling water and transferred to 75 gram of water at an initial temperature of 18C in an insulated container with negligible specific heat capacity. If the...
-
a) "Investors face a trade-off between risk and expected return. Historical data confirm our intuition that assets with low degrees of risk should provide lower returns on average than do those of...
-
Given the following three planes: D: (x,y,z)=(0,-1,4)+ k(-2, 1, 2) D (x,y,z)=(2,-1,14)+m(1,0,5) 1.Prove that these two lines are secant and find their point of intersection 2. Find the equation of...
-
A Watson Wyatt Worldwide survey showed that 58% of all Hispanic Americans are satisfied with their salary. Suppose a researcher randomly samples 27 Hispanic American workers and asks whether they are...
-
In each of the following independent cases, document the system using whatever technique(s) your instructor specifies. a. Dreambox Creations (www.dreamboxcreations.com/) in Diamond Bar, California,...
-
For each of the compounds below, locate the pattern we just learned (lone pair next to a Ï bond) and draw the appropriate resonance structure: a. b. c. d. e. f. g. h. NH2
-
Draw the resonance structure(s) for each of the compounds below: a. b. c. d.
-
The formalism of the Youngs modulus is sometimes used to calculate the reversible work involved in extending or compressing an elastic material. Assume a force F is applied to an elastic rod of...
-
A juice manufacturer produces and sells two fruit beverages, cranapple and apple- berry. One gallon of cran-apple beverage is made from three quarts of cranberry juice and one quart of apple juice;...
-
In 1991, Geoffrey A. Moore, a lecturer and management consultant, wrote an influential book titled Crossing the I Chasm. The book became an instant must-read for managers, entrepreneurs, and...
-
Understand the table 12 and its impact on answers. Supporting document 5 is below: Understand the table detached from the health and safety executive site HSE UK. Noise and health surveillance 4 (a)...

Study smarter with the SolutionInn App


