There are five hydrocarbon compounds (compounds of C and H) that have the formula C 6 H
Question:
There are five hydrocarbon compounds (compounds of C and H) that have the formula C6H14. (These are isomers; they differ in the way that C and H atoms are attached. All are liquids at room temperature but have slightly different densities 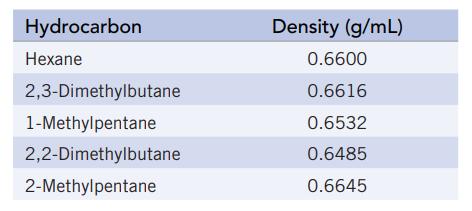
(a) You have a pure sample of one of these hydrocarbons, and to identify it you decide to measure its density. You determine that a 5.0-mL sample (measured in a graduated cylinder) has a mass of 3.2745 g (measured on an analytical balance). Assume that the accuracy of the values for mass and volume is plus or minus one (±1) in the last significant figure. What is the density of the liquid?
(b) Can you identify the unknown hydrocarbon based on your experiment?
(c) Can you eliminate any of the five possibilities based on the data? If so, which one(s)?
(d) You need a more accurate volume measurement to solve this problem, and you redetermine the volume to be 4.93 mL. Based on this new information, what is the unknown compound?
Step by Step Answer:

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel





