Verify that the atomic weight of lithium is 6.94, given the following information: 6 Li, mass =
Question:
Verify that the atomic weight of lithium is 6.94, given the following information:
6Li, mass = 6.015121 u; percent abundance = 7.50%
7Li, mass = 7.016003 u; percent abundance = 92.50%
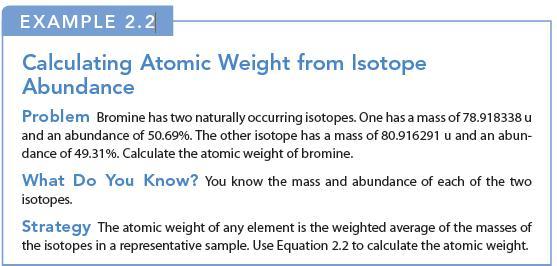

Transcribed Image Text:
EXAMPLE 2.2 Calculating Atomic Weight from Isotope Abundance Problem Bromine has two naturally occurring isotopes. One has a mass of 78.918338 u and an abundance of 50.69%. The other isotope has a mass of 80.916291 u and an abun- dance of 49.31%. Calculate the atomic weight of bromine. What Do You Know? You know the mass and abundance of each of the two isotopes. Strategy The atomic weight of any element is the weighted average of the masses of the isotopes in a representative sample. Use Equation 2.2 to calculate the atomic weight.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
Here to two isotope of Li is given Each natural abundance is different both mix and g...View the full answer

Answered By

Elias Gichuru
am devoted to my work and dedicated in helping my clients accomplish their goals and objectives,providing the best for all tasks assigned to me as a freelancer,providing high quality work that yields high scores.promise to serve them earnestly and help them achieve their goals.i have the needed expertise,knowledge and experience to handle their tasks.
4.80+
325+ Reviews
859+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Verify that the atomic weight of magnesium is 24.31, given the following information: 24 Mg, mass = 23.985042 u; percent abundance = 78.99% 25 Mg, mass = 24.985837 u; percent abundance = 10.00% 26...
-
Thallium has two stable isotopes, 203 Tl and 205 Tl. Knowing that the atomic weight of thallium is 204.4, which isotope is the more abundant of the two? EXAMPLE 2.2 Calculating Atomic Weight from...
-
Europium has two stable isotopes, 151 Eu and 153 Eu, with masses of 150.9197 u and 152.9212 u, respectively. Calculate the percent abundances of these isotopes of europium. EXAMPLE 2.2 Calculating...
-
Sherman Co. began operations on January 1, 2015, and completed several transactions during 2015 and 2016 that involved sales on credit, accounts receivable collections, and bad debts. These...
-
Describe seven principles for a user-centered interface design.
-
Discuss this statement: "An understanding of the causes and consequences of performance ambiguity is central to the issue of organizational design in multinational firms."
-
Cavett Problem. A process having multiple recycle loops formulated by R.H. Cavett [Proc. Am. Petrol. Inst., 43, 57 (1963)] has been used extensively to test tearing, sequencing, and convergence...
-
Valient Online Products is considering adopting the balanced scorecard and has compiled the following list of possible performance measures. Select the balanced scorecard perspective that best...
-
Describe a situation where you were responsible for getting others to make a change. What role did you play and what actions did you take? What was the outcome? If you had to do it again, would you...
-
Gallium has two naturally occurring isotopes, 69 Ga and 71 Ga, with masses of 68.9257 u and 70.9249 u, respectively. Calculate the percent abundances of these isotopes of gallium. EXAMPLE 2.2...
-
Name and describe the composition of the three hydrogen isotopes.
-
Determine the taxable gift for each of the following transfers made in 2018. a. In February, Cynthia transferred $200,000 into a revocable trust. In October, the trustee distributes $18,000 of income...
-
Super PB is the best-selling peanut butter on the market. A new peanut butter has come to market at half the price of Super PB and is affecting the demand for Super PB. Which determinant of demand is...
-
2x 11 8 14. Find the roots of x-2 x x-2x
-
what is the foundation of a successful virtual team?
-
What are the consequences of respiratory disorders, such as chronic obstructive pulmonary disease (COPD) and asthma, on gas exchange efficiency and overall physiological homeostasis ?
-
1. At the instant shown, the 4 lb. collar is moving up the rod at 6 ft/s. Determine the speed of the collar when it reaches point O if the spring has a stiffness k 50 lb./ft and an unstretched length...
-
You are helping a manufacturing firm decide whether it should invest in a new plant. The initial investment is expected to be $50 million, and the plant is expected to generate after-tax cash flows...
-
(a) Use integration by parts to show that (b) If f and g are inverse functions and f' is continuous, prove that (c) In the case where f and t are positive functions and b > a > 0, draw a diagram to...
-
Rewrite the van der Waals equation using the molar volume rather than V and n.
-
Propose a plausible synthesis for each of the following transformations: (a) (b) (c) (d)
-
Calculate w for the adiabatic expansion of 2.50 mol of an ideal gas at an initial pressure of 2.25 bar from an initial temperature of 450. K to a final temperature of 300. K. Write an expression for...
-
The cash account for the Justice Company at June 30, 20X8 indicated a balance of $5900. The bank statement indicated a balance of $5800 on June 30, 20X8. Comparing the bank statement and the...
-
The following information is provided for Roberts Company as of June 30, 20X8. 1. Bank Balance: $8638 2. Deposits in transit: $2200 3. Service charges: $40 4. Book Balance: $9700 5. First Bank...
-
The following selected accounts and their current balance for Peoples Inc. for the year ended December 31, 20X8. Additional information: Current portion of the Notes Payable: $ 17,625; additional...

Study smarter with the SolutionInn App


