What volume of 0.109 M HNO 3 , in milliliters, is required to react completely with 2.50
Question:
What volume of 0.109 M HNO3, in milliliters, is required to react completely with 2.50 g of Ba(OH)2?
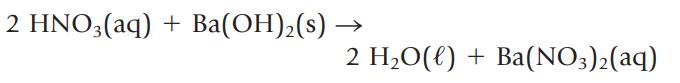
Transcribed Image Text:
2 HNO3(aq) + Ba(OH)₂(s) - 2 H₂O(l) + Ba(NO3)2(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
To determine the volume of 0109 M HNO3 required to react completely with 250 g of BaOH2 you can use ...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
What volume of 0.750 M Pb(NO 3 ) 2 , in milliliters, is required to react completely with 1.00 L of 2.25 M NaCl solution? The balanced equation is Pb(NO3)2(aq) + 2 NaCl(aq) PbCl(s) + 2 NaNO3(aq)
-
1. What mass of H2 should be produced by the reaction of Al with 75.0 mL of 2.95M HCl? 2Al(s) + 6HCl(aq) 2AlCl3(aq) + 3H2 (g). ln the lab, 0.15g H2 was collected. What is the % yield of the...
-
Marnie wants to save up $250,000 to pay cash for a home purchase 15 years from now. If her investment can carn 6.1% compounded monthly and she intends to grow each payment by 0.25%, what will be her...
-
Suggest two strategies for the company to minimise the impact of the strike on business operation?
-
Describe three recent situations in which you were directly affected by poor product or service quality.
-
Williams & Sons last year reported sales of $12 million, cost of goods sold (COGS) of $10 million, and an inventory turnover ratio of 2. The company is now adopting a new inventory system. If the new...
-
Von Krmn assumed a cubic profile for the integral momentum analysis over a flat plate. Since a cubic has four constants, four conditions were used. (i) \(V_{x}=0\) at \(y=0\). (ii)...
-
The Comparative balance sheets for Karidis Ceramics, Inc., for December 31, 2012 and 2011, are presented on the next page. During 2012, the company had net income of $96,000 and building and...
-
After examining the opportunity set you realize that you can invest in a portfolio consisting of the bond fund and the large-cap stock fund that will have exactly the same standard deviation as the...
-
What mass of Na 2 CO 3 , in grams, is required for complete reaction with 50.0 mL of 0.125 M HNO 3 ? NaCO3(aq) + 2 HNO3(aq) 2 NaNO3(aq) + CO(g) + HO(l)
-
What is the hydronium ion concentration of a 1.2 10 4 M solution of HClO 4 ? What is its pH?
-
Evaluate using the identity a-b = (a - b)(a + ab + b)
-
Pony Corp wants to issue debt with one year maturity. Adding debt will increase the cost of bankruptcy, but also increase the tax benefit of debt. Pony has calculated the following estimates of the...
-
Answer the Following questions by using Financial Statement Growth vs External financing Fixed Debt-Equity Ratio vs Internal Growth Rate Dividend Payout Ratio
-
Suppose an array holds a sequence of N unique numbers that are sorted in decreasing order. If we move the last P numbers (P
-
def my_sum (L): X=0 y = 1 n i = 0 len (L). while x + y
-
Assume that you have an array of integers C with indexes 1...n. Consider the following recurrence: A(i) = | min(C[i 1] + A(i 1), C[i 2] + A(i-2)) - min(C[i 1] + A(i 1), C[i + 1] + A(i+1))...
-
A study by Peter D. Hart Research Associates for the Nasdaq Stock Market revealed that 43% of all American adults are stockholders. In addition, the study determined that 75% of all American adult...
-
A bubble-point liquid feed is to be distilled as shown in Figure. Use the Edmister group method to estimate the mole-fraction compositions of the distillate and bottoms. Assume initial overhead and...
-
When benzene is treated with I 2 in the presence of C u Cl 2 , iodination of the ring is achieved with modest yields. It is believed that C u Cl 2 interacts with I 2 to generate I + , which is an...
-
The interior of a refrigerator is typically held at 36F and the interior of a freezer is typically held at 0.00F. If the room temperature is 65F, by what factor is it more expensive to extract the...
-
Using your results from Problem P5.7, calculate q, ÎU, and ÎH for each step in the cycle and for the total cycle described in Figure 5.2. Figure 5.2 Isothermal expansion Pa Adiabatic cold...
-
es As of June 30, Year 2, the bank statement showed an ending balance of $16,554. The unadjusted Cash account balance was $15,305. The following information is available: 1. Deposit in transit,...
-
Rick Hall owns a card shop, Hall's Cards. The following cash information is available for the month of August, Year 3. As of August 31, the bank statement shows a balance of $16,140. The August 31...
-
Ethics Case The Siesta Hotel is owned by several individuals who also manage its oper- ations. These management team members are individuals of high integrity with a strong commitment to guest...

Study smarter with the SolutionInn App


