What volume of 0.955 M HCl, in milliliters, is required to titrate 2.152 g of Na 2
Question:
What volume of 0.955 M HCl, in milliliters, is required to titrate 2.152 g of Na2CO3 to the equivalence point? 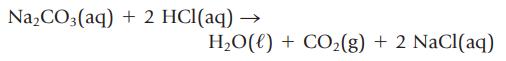
Transcribed Image Text:
Na₂CO3(aq) + 2 HCl(aq) – H₂O(l) + CO₂(g) + 2 NaCl(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
To determine the volume of 0955 M HCl required to titrate 2152 g of Na2CO3 to the equivalence po...View the full answer

Answered By

Ishrat Khan
Previously, I have worked as an accounting scholar at acemyhomework, and have been tutoring busines students in various subjects, mostly accounting. More specifically I'm very knowledgeable in accounting subjects for college and university level. I have done master in commerce specialising in accounting and finance as well as other business subjects.
5.00+
135+ Reviews
427+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
If 38.55 mL of HCl is required to titrate 2.150 g of Na 2 CO 3 according to the following equation, what is the concentration (mol/L) of the HCl solution? NaCO3(aq) + 2 HCl(aq) - 2 NaCl(aq) + CO(g) +...
-
What volume of 0.812 M HCl, in milliliters, is required to titrate 1.45 g of NaOH to the equivalence point? NaOH(aq) + HCl(aq) HO(l) + NaCl(aq)
-
You have 0.954 g of an unknown acid, H 2 A, which reacts with NaOH according to the balanced equation If 36.04 mL of 0.509 M NaOH is required to titrate the acid to the second equivalence point, what...
-
Why program planning is important in public health leadership?
-
A production manager at Ultra Clean Dishwashing Company is monitoring the quality of the companys production process. There has been concern relative to the quality of the operation in accurately...
-
When dishes are not properly rinsed after washing, different colors are reflected from their surfaces. Explain.
-
By considering the rotational equilibrium of a fluid mass element, show that \(\tau_{x y}=\tau_{y x}\).
-
When Lyle was hired, she was told that typing speed was extremely important to the position. At the time, she maintained that she could type eighty words per minute, so she was not given a typing...
-
What is performance management systems? Do the companies where you (or others you know) have worked used performance management systems rather than performance appraisal systems? If yes, what kind of...
-
Potassium hydrogen phthalate, KHC 8 H 4 O 4 , is used to standardize solutions of bases. The acidic anion reacts with strong bases according to the following net ionic equation: If a 0.902-g sample...
-
What volume of 0.125 M oxalic acid, H 2 C 2 O 4 , is required to react with 35.2 mL of 0.546 M NaOH? HCO4(aq) + 2 NaOH(aq) NaCO4(aq) + 2 HO(l)
-
Made by the Tootsie Industries of Chicago, Illinois, Mason Dots is a gumdrop candy. A box was opened by the authors and was found to contain the following number of gumdrops: Orange 9 Lemon 8...
-
Simplify log(8)(log(5x)) = 0
-
The lengths of pregnancies in a small rural village are normally distributed with a mean of 266 days and a standard deviation of 13 days. In what range would you expect to find the middle 68% of most...
-
When Webflicks increased monthly subscription fees from $15 to $17, 16% of its subscribers switched to alternative providers. (a) Provide an estimate of Webflicks demand elasticity. (b) Assuming that...
-
Based on what you know about angular velocity and acceleration, explain what the horizontal acceleration of the ball would be using the measurements of the arm for reference. What could be a...
-
Restorative Justice is a controversial form of punishment. Do you believe Restorative Justice will reduce crime? Please explain in details.
-
When a receivable is written off under the allowance method, how does it affect the net realizable value shown on the balance sheet?
-
Represent each of the following combination of units in the correct SI form using an appropriate prefix: (a) m/ms, (b) k m, (c) k s /mg, and (d) k m N.
-
Two vessels of equal volume, pressure, and temperature both containing Ar are connected by a valve. What is the change in entropy when the valve is opened, allowing mixing of the two volumes? Is S...
-
Without using equations, explain why S for a liquid or solid is dominated by the temperature dependence of S as both P and T change.
-
Solid methanol in thermal contact with the surroundings is reversibly melted at the normal melting point at a pressure of 1 atm. Are S, S surroundings , and S total positive, negative, or zero?...
-
Find recent (within a few years) articles from the web, business magazines, etc., explaining how the company used either (or both) DSS or AI effectively. Explain what AI and DSS are How has this...
-
You have noticed a downward trend in engagement levels of the employees over the past 5 years. You recognize that in order to be convincing, you will need to provide a strategy that is data-driven...
-
1. Pick a time in life when a person can approach a project in a systems-thinking way. How would systems thinking help them? 2. Define a causal-loop logic in relation to corporate objectives. Why is...

Study smarter with the SolutionInn App


