If 38.55 mL of HCl is required to titrate 2.150 g of Na 2 CO 3 according
Question:
If 38.55 mL of HCl is required to titrate 2.150 g of Na2CO3 according to the following equation, what is the concentration (mol/L) of the HCl solution? 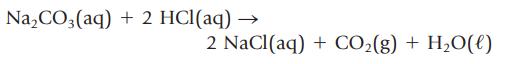
Transcribed Image Text:
Na₂CO3(aq) + 2 HCl(aq) - 2 NaCl(aq) + CO₂(g) + H₂O(l)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
To calculate the concentration of the HCl solution we can use the following ...View the full answer

Answered By

Poonam Chaudhary
I have 15 month+ Teaching Experience
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
When an acid reacts with a base: 1) This is a neutralization reaction 2) Pink color will appear in the resulting solution 3) Both of the answers are correct 4) None of the answers is correct QUESTION...
-
The cancer drug cisplatin, Pt(NH 3 ) 2 Cl 2 , can be made by reacting (NH 4 ) 2 PtCl 4 with ammonia in aqueous solution. Besides cisplatin, the other product is NH 4 Cl. (a) Write a balanced equation...
-
1. What mass of H2 should be produced by the reaction of Al with 75.0 mL of 2.95M HCl? 2Al(s) + 6HCl(aq) 2AlCl3(aq) + 3H2 (g). ln the lab, 0.15g H2 was collected. What is the % yield of the...
-
A) Suppose you wanted to make a photoconductor that interacts in the visible, green light range (500-570 nm). Which of the following semiconductors would be your best choice, and why? Si, AIP, InAs,...
-
The Awake Coffee Company produces gourmet instant coffee. The company wants to be sure that the average fill of coffee containers is 12.0 ounces. To make sure the process is in control, a worker...
-
A pulse of red light and a pulse of blue light enter a glass block at the same time normal to its surface. Strictly speaking, after passing through the block, which pulse exits first?
-
The stream function for a given two-dimensional flow field is \[ \psi=5 x^{2} y-(5 / 3) y^{3} \] Determine the corresponding velocity potential.
-
Cyclone Software Co. is trying to establish its optimal capital structure. Its current capital structure consists of 25% debt and 75% equity; however, the CEO believes that the firm should use more...
-
Discuss the cultural differences between China and the United States. What would be the biggest adjustments I might need to make if working there as an expatriate? What are the most...
-
Potassium hydrogen phthalate, KHC 8 H 4 O 4 , is used to standardize solutions of bases. The acidic anion reacts with strong bases according to the following net ionic equation: If a 0.902-g sample...
-
What volume of 0.955 M HCl, in milliliters, is required to titrate 2.152 g of Na 2 CO 3 to the equivalence point? NaCO3(aq) + 2 HCl(aq) HO(l) + CO(g) + 2 NaCl(aq)
-
Construct a translation of quadrilateral ABCD, shown below, using translation vector v. Show the quadrilateral in the positions both before and after the translation. A B D
-
Explain Independent contractors, and the duties performed by them.
-
With a view to avoiding missing out the opportunities to enter into beneficial business transactions, list the Rules of Offer and Acceptance that should always be observed.
-
The Snoop'n'trude Security Service has searched its employees' lockers several times without a warrant. The employees' lawyer brings a class action lawsuit based on violation of the Fourth Amendment....
-
Four years ago, Chaplin Inc. purchased a new machine with a 5-year expected life for $35,500. On its current balance sheet, Chaplin lists accumulated depreciation on the machine at $28,400. What is...
-
Corporations are important because of advantages created by the unique characteristics of the corporate structure of ownership. Which of the following makes buying shares in a corporation attractive...
-
How does the percent-of-sales method compute bad debts expense?
-
The manager of a local convenience store is expanding his line of small toy items. To price these new items, the manager is looking at the prices being charged by competing retailers in his area. For...
-
A compound with molecular formula C 11 H 14 O 2 exhibits the following spectra ( 1 H NMR, 13 C NMR, and IR). Identify the structure of this compound. Proton NMR 11 Chemical Shift (ppm) Carbon NMR...
-
Is the equation valid for an ideal gas? Tf PV; -V;) T; Cy dT Lav = C, n2v, -v) %3D AS =
-
Why is the efficiency of a Carnot heat engine the upper bound to the efficiency of an internal combustion engine?
-
Describe a potential case scenario involving an MSN-prepared nurse in leadership and management. Within the scenario, address the concern conflict between team members that can impact your future MSN...
-
Answer all parts in full detail. Part 1 Identify 3-5 activities that you would like to include in the professional development plan as a public-sector employee. Discuss at least 2 activities from...
-
Explain the PMO organizational structure, the rationale for its make-up, and how this configuration can be used to accomplish the goals of the project. Will project managers be included in the PMO,...

Study smarter with the SolutionInn App


