When an electric current is passed through an aqueous solution of NaCl, the valuable industrial chemicals H
Question:
When an electric current is passed through an aqueous solution of NaCl, the valuable industrial chemicals H2(g), Cl2(g), and NaOH are produced. 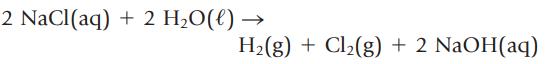
What mass of NaOH can be formed from 15.0 L of 0.35 M NaCl? What mass of chlorine is obtained?
Transcribed Image Text:
2 NaCl(aq) + 2 H₂O(l) - H₂(g) + Cl₂(g) + 2 NaOH(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
To find the mass of NaOH formed and the mass of chlorine obtained you can use the given information ...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
1) Emily wants to buy a computer that has a price of $1500, and she decides to pay for it with installments over 5 years. If the interest rate is 10 %, what is the monthly payment? 7) Suppose Crystal...
-
Indium(III) phosphide is a semiconducting material that has been frequently used in lasers, light-emitting diodes (LED), and fiberoptic devices. This material can be synthesized at 900. K according...
-
When an electric current is passed through molten sodium chloride, a. sodium metal is deposited at the positive electrode b. sodium ions are deposited at the positive electrode c. chlorine gas is...
-
What is the type of the expressions computed on these two lines? 4 > 5 print (4>5)
-
Describe a quality control chart and how it can be used. What are upper and lower control limits? What does it mean if an observation falls outside the control limits?
-
Hatfield Medical Supply's stock price had been lagging its industry averages, so its board of directors brought in a new CEO, Jaiden Lee. Lee had brought in Ashley Novak, a finance MBA who had been...
-
A cubic approximation is commonly used in conjunction with the von Krmn momentum integral. An alternative form is the sine function: \[v_{x}=\alpha \sin (b y)\] What should the constants \(\alpha\)...
-
The condensed financial statements for OIL Inc. and ERS Company for the year ended December 31, Year 5, follow: On December 31, Year 5, after the above figures were prepared, OIL issued $240,000 in...
-
A business has a cost of equity of 9.5 percent and a pretax cost of debt of 5.4 percent. The debt-equity ratio is 1.15 and the tax rate is 25 percent. What is the unlevered cost of capital?
-
Hydrazine, N 2 H 4 , a base like ammonia, can react with sulfuric acid. What mass of hydrazine reacts with 250. mL of 0.146 M H 2 SO 4 ? 2 NH(aq) + HSO4(aq) 2 NH5+ (aq) + SO4- (aq)
-
What mass of Na 2 CO 3 , in grams, is required for complete reaction with 50.0 mL of 0.125 M HNO 3 ? NaCO3(aq) + 2 HNO3(aq) 2 NaNO3(aq) + CO(g) + HO(l)
-
Find the domain and range of the function. (x) = 1x 2
-
15. You would like to have a balance of $600,000 at the end of 15 years from monthly savings of $900. If your returns are compounded monthly, what is the APR you need to meet your goal? Enter your...
-
4. Suppose that you estimate the following cost function for your company, which is a firm operating in a perfectly competitive market: TC=100Q-4Q 2 +Q 3 The market demand and supply equations are as...
-
You are given TWO plans to join a professional association. an X requires a payment of $185 starting in year 0, and three other equal payments of $185, for years ONE, TWO and THREE. Plan Y requires a...
-
A bond paying 5% coupons semiannually and YTM of 4.5% and 12 years to maturity. If the last interest payment was made 30 days ago, assuming 364 days a year, calculate the dirty price of the bond. If...
-
December 31, 2030 Accounts Debit Credit Cash Accounts Receivable 105,800 14,600 Allowance for Bad Debts Inventory Prepaid Advertising Supplies Accounts Payable Unearned Revenue Long-Term Note Payable...
-
A study by Becker Associates, a San Diego travel consultant, found that 30% of the traveling public said that their flight selections are influenced by perceptions of airline safety. Thirty-nine...
-
6. (Potential Energy and Conservation of Energy) What should be the spring constant k of a spring designed to bring a 1200-kg car to rest from a speed of 95 km/h so that the occupants undergo a...
-
Propose a plausible synthesis for the following transformation. CH3 CH3 CH3
-
Calculate S for the isothermal compression of 1.75 mol of Cu(s) from 2.15 bar to 1250. bar at 298 K. = 0.492 10 4 K 1 , = 0.78 10 6 bar 1 , and the density is 8.92 g cm 3 . Repeat the calculation...
-
Calculate S for the reaction 3H 2 (g) + N 2 (g) 2NH 3 (g) at 725 K. Omit terms in the temperature-dependent heat capacities higher than T 2 /K 2 .
-
INSTRUCCIONES GENERALES: Razonara los problemas y resulvalos en las hojas anexas. 1. La empresa importadora, nos enva de Miami, estados unidos, mercancas amparadas con factura lo siguiente 146...
-
Examine the summary of the balance of payments of the United States for 2021. Compute and discuss the balance on the current account and its subaccounts. A Summary of the U.S. Balance of Payments for...
-
Gallatin Carpet Cleaning is a small, family-owned business operating out of Bozeman, Montana. For its services, the company has always charged a flat fee per hundred square feet of carpet cleaned....

Study smarter with the SolutionInn App


