A Lewis structure for the oxalate ion is shown below. (One or more other resonance forms are
Question:
A Lewis structure for the oxalate ion is shown below. (One or more other resonance forms are also possible.)
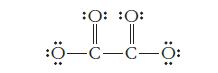
What is the correct charge on the oxalate ion? What type of orbital hybridization is expected for each of the carbon atoms in this structure? How many sigma bonds and how many pi bonds does the structure contain?
Transcribed Image Text:
-C-C- :O: :0:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Based on the valence of each atom a neutral molecule ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
The azide ion, N3-, is linear with two N-N bonds of equal length, 1.16 Ã. (a) Draw a Lewis structure for the azide ion. (b) With reference to Table 8.5, is the observed bond length consistent...
-
Methyl cyanoacrylate, C 5 H 5 NO 2 , is the compound commonly sold as super glue. The glue works through a polymerization reaction, in which molecules of methyl cyanoacrylate form strong chemical...
-
The lactic acid molecule, CH3CH (OH) COOH, gives sour milk its unpleasant, sour taste. (a) Draw the Lewis structure for the molecule, assuming that carbon always forms four bonds in its stable...
-
The mass of the crane?s boom is 9000 kg. Its weight acts at?G. The sum of the moments about?P?due to the boom?s weight, the force exerted at?B?by the cable?AB,?and the force exerted at?C?by the...
-
Real options can be analyzed using a scenario approach with decision trees or using the Black-Scholes Option Pricing Model. What are the pros and cons of the two approaches? Is one procedure better...
-
Walshs Fruit Company contracts with growers in Ohio, Pennsylvania, and New York to purchase grapes. The grapes are processed into juice at the farms and stored in refrigerated vats. Then the juice is...
-
Define a sequence of correlated random numbers \[ s_{k}=\alpha s_{k-1}+(1-\alpha) r_{k} \] where \(r_{k}\) is a unit-variance, uncorrelated, Gaussian pseudorandom number while \(0
-
Current E&P Computation. Water Corporation reports $500,000 of taxable income for the current year. The following additional information is available: For the current year, Water reports an $80,000...
-
Provide three comparisons between hierarchical, network and relational database models. You can present your answer in the table. (b) The relational database model is the most widely used database....
-
Nearly all of the other elements form binary compounds with hydrogen. Based on the electronegativity values shown in Figure 7.7, with what category of elements will hydrogen form bonds in which the...
-
The following molecules have similar formulas, but each has a different shape. Draw Lewis structures for these compounds and then determine their molecular shapes. (a) SiF 4 , (b) KrF 4 , (c) SeF 4
-
Ramsden Manufacturing sold merchandise with a gross price of $25,000 to Garner's Hardware Store. Ramsden offered terms of 3/10, n/30. Required: Prepare the necessary journal entries to record the...
-
2. The rotating-cylinder viscometer shown in the figure below shears the fluid in a narrow clear- ance Ar. The torque (M) required to rotate the cylinder at an angular velocity (2) is measured and...
-
The process of risk management requires organizations to understand risk to operations. This requires analysis and planning. 1. Define and explain Risk. 2. What is Risk management and the Risk...
-
The number of students using a new cell phone application doubles every seven days. The initial number of users is 100 How many students will use the application after t days?
-
An investor buys an annuity with payments of principal and interest of $500 per quarter for 13 years. Interest is at the effective rate of 7% per annum How much interest does the investor receive in...
-
solve problems of "LinkedList.java", problems are in comments of "LinkedList.java" Please showing the output There are some tested method in "LinkedListTest.java" (Please don't modify "Account.java"...
-
The following income statement does not reflect intraperiod tax allocation. Required: Recast the income statement to reflect intraperiod tax allocation. The company's tax rate is40%. INCOME STATEMENT...
-
Assume you are the accountant for Catalina Industries. John Catalina, the owner of the company, is in a hurry to receive the financial statements for the year ended December 31, 20X1, and asks you...
-
Show using an example that the following two formulations of the Pauli exclusion principle are equivalent: a. Wave functions describing a many-electron system must change sign under the exchange of...
-
Calculate the terms that can arise from the configuration np 1 np 1 , n n. Compare your results with those derived in the text for np 2 . Which configuration has more terms and why?
-
Calculate the terms that can arise from the configuration np 1 np 1 , n n. Compare your results with those derived in the text for np 2 . Which configuration has more terms and why?
-
What are the contemporary debates and revisions within modernization theory, considering the changing dynamics of globalization, digital technology, and environmental sustainability, and their impact...
-
Specific, concrete ways an organization can support and encourage the four components of individual creativity that can lead to innovation. Do you think organizational support is sufficient to drive...
-
ious Write an equation modeled by the envelopes and counters, and then solve the equation. (Hint: Let x be the contents of the envelope.) Provide your answer below: equation: x + = solution: x=

Study smarter with the SolutionInn App


