Although ammonia is made in enormous quantities by the Haber-Bosch process, sulfuric acid is made in even
Question:
Although ammonia is made in enormous quantities by the Haber-Bosch process, sulfuric acid is made in even greater quantities by the contact process. A simplified version of this process can be represented by these three reactions:
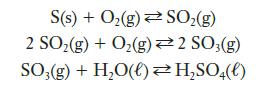
(a) Use tabulated data to calculate ΔH° for each reaction.
(b) Which reactions are exothermic? Which are endothermic?
(c) In which of the reactions does entropy increase? In which does it decrease? In which does it stay about the same?
(d) For which reaction(s) do low temperatures favor formation of products?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





