Copper and iron (generally in the form of steel) are two of the many metals used in
Question:
Copper and iron (generally in the form of steel) are two of the many metals used in designing machines.
(a) Using standard reduction potentials, identify the anode and the cathode and determine the cell potential for a galvanic cell composed of copper and iron. Assume standard conditions.
(b) We can also construct a galvanic cell using copper and silver. Confirm that the potential of the following galvanic cell is 0.462 V:
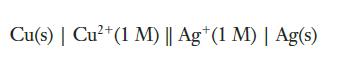
Strategy
We must interpret the nature of an electrochemical system based on the information available in a table of standard reduction potentials. With two half-reactions there are only two possible outcomes—and one outcome yields a negative value for the cell potential. Because we know that a galvanic cell cannot have a negative E° value, we must determine the combination of half-reactions that provides a positive value for E°.
Step by Step Answer:

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme





