Ammonia can react with oxygen to produce nitric oxide and water: If the rate at which ammonia
Question:
Ammonia can react with oxygen to produce nitric oxide and water:
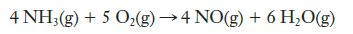
If the rate at which ammonia is consumed in a laboratory experiment is 4.23 × 10–4 mol L–1 s–1, at what rate is oxygen consumed? At what rate is NO produced? At what rate is water vapor produced?
Transcribed Image Text:
4 NH3(g) + 5 O₂(g) →4 NO(g) + 6 H₂O(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The rate of oxygen consumption i...View the full answer

Answered By

Anurag Agrawal
I am a highly enthusiastic person who likes to explain concepts in simplified language. Be it in my job role as a manager of 4 people or when I used to take classes for specially able kids at our university. I did this continuously for 3 years and my god, that was so fulfilling. Sometimes I've skipped my own classes just to teach these kids and help them get their fair share of opportunities, which they would have missed out on. This was the key driver for me during that time. But since I've joined my job I wasn't able to make time for my passion of teaching due to hectic schedules. But now I've made a commitment to teach for at least an hour a day.
I am highly proficient in school level math and science and reasonably good for college level. In addition to this I am especially interested in courses related to finance and economics. In quest to learn I recently gave the CFA level 1 in Dec 19, hopefully I'll clear it. Finger's crossed :)
4.80+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
The source of oxygen that drives the internal combustion engine in an automobile is air. Air is a mixture of gases, principally N2 (~79%) and O2 (~20%). In the cylinder of an automobile engine,...
-
The Ostwald process is used commercially to produce nitric acid, which is, in turn, used in many modern chemical processes. In the first step of the Ostwald process, ammonia is reacted with oxygen...
-
In 1991 it was discovered that nitrous oxide (N2O) is produced in the synthesis of nylon. This compound, which is released into the atmosphere, contributes both to the depletion of ozone in the...
-
Several industries located along the Ohio River discharge a toxic substance called carbon tetrachloride into the river. The state Environmental Protection Agency monitors the amount of carbon...
-
During January 2011, Doe Corp. agreed to sell the assets and product line of its Hart division. The sale was completed on January 15, 2012; on that date, Doe recognized a gain on disposal of...
-
Kazaam Company, a merchandiser, recently completed its calendar-year 2011 operations. For the year, (1) All sales are credit sales, (2) All credits to Accounts Receivable reflect cash receipts from...
-
Refer to the information in Exercise 17-1. Assume that the following information is available for the companys two products for the first quarter of 2017. Required Compute activity rates for each...
-
Via Gelato is a popular neighborhood gelato shop. The company has provided the following data concerning its operations: While gelato is sold by the cone or cup, the shop measures its activity in...
-
19. Write your first derivative of the following function in the space on the answer sheet. Y 2X2+5X3-X+10 20. Write your first derivative of the following function in the space on the answer sheet....
-
The following data were obtained in the decomposition of H 2 O 2 (aq) to O 2 (g) and H 2 O(). The rate at which oxygen gas was produced was measured. (No oxygen was present initially.) (a) Calculate...
-
The reaction for the Haber process, the industrial production of ammonia, is: Assume that under certain laboratory conditions ammonia is produced at the rate of 6.29 10 5 mol L 1 s 1 . At what rate...
-
The most stable conformation of most aldopyranoses is one in which the largest group, the iCH2OH group, is equatorial. However, d-idopyranose exists primarily in a conformation with an axial --CH2OH...
-
When an asset is sold, the undepreciated capital cost in its asset class (or pool) is reduced by what is realized on the asset or by its original cost, whichever is less. This amount is called the?
-
Entered a 6 year loan on 1 march current year for the purchase of equipment that was required for income producing purpose. The borrowing cost assosiated with loan were. 750. What is deductible...
-
A 3.00-kg ball, moving to the right at a velocity of +3.50 m/s on a frictionless table, collides head-on with a stationary 7.90-kg ball. Find the final velocities of (a) the 3.00-kg ball and of (b)...
-
Which alternative is better? probability of soft kill of each three alternatives against each of three threats. in addition, the relative frequency of attacks is 40% by country 1, 50% by country 2,...
-
How do protein conformational changes, such as allosteric transitions, modulate enzymatic activity and cellular signaling cascades?
-
Vice Oil received the following selected information from its pension plan trustee concerning the operation of the companys defined-benefit pension plan for the year ended December 31, 2014. The...
-
The graph of the sequence whose general term is an = n - 1 is which of the following? [8.1] A. B. TITTT 3-2-1 23.45 2.3.4
-
Using the data in the following table, calculate the pI of the following amino acids. (a) Aspartic acid (b) Leucine (c) Lysine (d) Proline THE PK,VALUES FOR TWENTY NATURALLY OCCURRING AMINO ACIDS...
-
The OH group on the side chain of serine is not deprotonated at a pH of 12. However, the OH group on the side chain of tyrosine is deprotonated at a pH of 12. This can be verified by inspecting the...
-
Explain why it is inappropriate to use a chiral catalyst in the preparation of glycine.
-
What effect has legal changes that allow more consolidation of media under major corporations had? Do we need new laws limiting media ownership in the U.S.? Why? Use specific examples in your...
-
Should criminal justice agencies maintain a social media presence? Should police departments have a dedicated social media unit? How have social media sites assisted law enforcement in fighting and...
-
What are scruples? Describe a situation where you demonstrated scruples. What are the dangers of social media for law enforcement officers? In what ways do police departments regulate social media...

Study smarter with the SolutionInn App


