Calcium carbonate (limestone, CaCO 3 ) dissolves in hydrochloric acid, producing water and carbon dioxide. An unbalanced
Question:
Calcium carbonate (limestone, CaCO3) dissolves in hydrochloric acid, producing water and carbon dioxide. An unbalanced net ionic equation for this reaction is given below. Balance it.
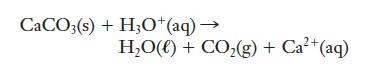
Transcribed Image Text:
CaCO3(s) + H3O+ (aq) → H,O() + CO,(g)+Ca+(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Balanced chemical equation ...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Calcium carbonate (limestone, CaCO 3 ) dissolves in hydrochloric acid, producing water and carbon dioxide as shown in the following unbalanced net ionic equation: Suppose 5.0 g of CaCO 3 is added to...
-
QUESTION 1 When propane undergoes complete combustion, the products are carbon dioxide and water.? ? ? ? __ C 3 H 8 (g) + __ O 2 (g) ? __ CO 2 (g) + __ H 2 O(g)What are the respective coefficients...
-
9. If a two-dimensional array is declared as int M[3][2]; and it has been initialized to all zero's, what will be the contents of this two-dimensional array after executing the code fragment below...
-
A 10.231-g sample of window cleaner containing ammonia was diluted with 39.466 g of water. Then 4.373 g of solution were titrated with 14.22 mL of 0.1063 M HCl to reach a bromocresol green end point....
-
A firm wishing to evaluate interest rate behavior has gathered data on the nominal rate of interest and on inflationary expectations for five U.S. Treasury securities, each having a different...
-
Assume the spot Swiss franc is $0.7000 and the six-month forward rate is $0.6950. What is the minimum price that a six-month American call option with a striking price of $0.6800 should sell for in a...
-
An often-ignored concept in breach of contract is the availability, if any, of the award of punitive damages. Often, cases incorporate both breach of contract and tort actions. The tort actions...
-
Consider the following four LP formulations. Using a graphical approach, determine (a) Which formulation has more than one optimal solution. (b) Which formulation is unbounded. (c) Which formulation...
-
8) What does the following method do? Rewrite it so it produces the same results but does not use recursion. public static boolean whoKnows (int arr, int i, int j) { if (i >= j) { return true; } else...
-
Methyl cyanoacrylate is the chemical name for the substance sold as Super Glue, and it has the chemical formula C 5 H 5 NO 2 . Calculate the number of molecules of this substance in a 1.0-ounce tube...
-
Many chemical reactions take place in the catalytic converter of a car. In one of these reactions, nitric oxide (NO)reacts with ammonia (NH 3 ) to give nitrogen (N 2 ) and water. Write a balanced...
-
Let X be the product of the numbers on two dice rolled. (a) Does X assume all values between 1 and 36? (b) Trying to provide all calculations in mind (they are easy), find P(X 36), P(X < 36), P(X ...
-
Draw the structures of all possible isomers of the compound with molecular formula C3H6O. Given that this compound produces a strong IR absorption at 1725cm-1 . Which isomer is most consistent with...
-
paid dividends at a rate of 2 rupees per share last year. The estimated growth of the company is approximately 5 % per share. Determine the cost of equity capital of the company.
-
Would you have any additional special separate recordkeeping for ITAR shipments (versus EAR)? Why or why not.
-
It was determined that there was fraud present at Husky Corporation, so you will be auditing the Accounts Receivable of Husky Corporation. The Accounts Receivable general ledger balance as of...
-
1. Using the tree implementation of disjoint sets answer the following questions. Proofs are not necessary for this question. (a) Describe a sequence of make-set and union operations that builds a...
-
We are examining a new project. We expect to sell 9,000 units per year at $35 net cash flow apiece for the next 10 years. In other words, the annual operating cash flow is projected to be $35 X 9,000...
-
A city maintains a solid waste landfill that was 12 percent filled at the end of Year 1 and 26 percent filled at the end of Year 2. During those periods, the government estimated that total closure...
-
Draw a mechanism for each of the following transformations: a. b. c. ,t Dilute H,SO,
-
In this problem, you calculate the error in assuming that ÎH o R is independent of T for the reaction 2CuO(s) 2Cu(s) + O 2 (g). The following data are given at 25°C:
-
If an alkene is protonated and the solvent is an alcohol rather than water, a reaction takes place that is very similar to acid-catalyzed hydration, but in the second step of the mechanism the...
-
Consider both a 12-month American and a 12-month European put option on a stock with So = $100, K = $90, and = 0.3. The risk-free rate is 5%, continuously compounded, for the entire 12-month period....
-
Bavarian Sausage just issued a 7-year 9% coupon bond. The face value of the bond is $1,000 and the bond makes annual coupon payments. If the bond is trading at $982.54, what is the bond's yield to...
-
Benton County is evaluating 3 new projects for future investment. The county uses a MARR of 6% to make financial decisions. *CFD not required. a. Find the benefit-cost ratio for the three projects....

Study smarter with the SolutionInn App


