Calcium sulfate is the essential component of plaster and sheet rock. Waste calcium sulfate can be converted
Question:
Calcium sulfate is the essential component of plaster and sheet rock. Waste calcium sulfate can be converted into quicklime, CaO, by reaction with carbon at high temperatures. The following two equations represent a sequence of reactions that might take place:
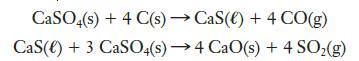
What mass of sulfur dioxide could be obtained from 1.250 kg of calcium sulfate?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





