In Example Problem 5.1, we considered a CH 4 storage tank with a volume of 49.0l. When
Question:
In Example Problem 5.1, we considered a CH4 storage tank with a volume of 49.0l. When empty, the tank has a mass of 55.85 kg, and when filled, its mass is 62.07 kg.
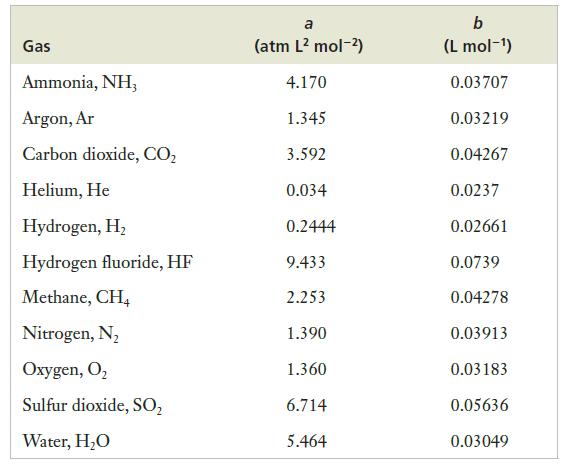
Calculate the pressure of CH4 in the tank at an ambient temperature of 21°C using both the ideal gas equation and the van der Waals equation. What is the percentage correction achieved by using the more realistic van der Waals equation?
Strategy We will use two different models to describe the same gas, and each model will be represented by its own equation. So we will do two independent calculations, solving for P in each case. We can find the mass of CH4 from the given data and then convert that mass into the number of moles by using the molar mass. And the van der Waals constants for CH4 can be found in Table 5.2. To calculate the percentage difference, we will divide the difference of the two numbers by the value for the ideal gas case.
Table 5.2.
Data from example 5.1
For homes or businesses in some rural areas, natural gas pipelines may not be available. In such cases, methane might be obtained in a compressed gas cylinder. A common laboratory cylinder of methane has a volume of 49.0 L and is filled to a pressure of 154 atm. Suppose that all of the CH4 from this cylinder is released and expands until its pressure falls to 1.00 atm. What volume would the CH4 occupy?
Strategy The problem involves a change in the conditions, here pressure and volume, of a sample of gas. We will want to use the ideal gas law because it is our model that relates the various properties of a gas to one another. So we begin by assuming that the gas will obey the ideal gas law under both the initial and final conditions. We will further assume that temperature is constant because we are not given any data to suggest otherwise. Because we are working with the same sample of gas throughout, we also know that the number of moles (n) is constant. Collect the constant terms together and work toward a solution.
Step by Step Answer:

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme





