The rate of the photodissociation of ozone in the example shown in Figure 11.7 may seem slow.
Question:
The rate of the photodissociation of ozone in the example shown in Figure 11.7 may seem slow. But it is actually tremendously faster than what we would see in the absence of ultraviolet light. The rate constant, k, for the thermal decomposition of ozone in the dark at 25°C is just 3 × 10–26 s–1. What is the half-life of ozone under these conditions?
Strategy We can see from the units of the rate constant that this is a first-order reaction. So, we can use the equation for the first-order half-life.
Figure 11.7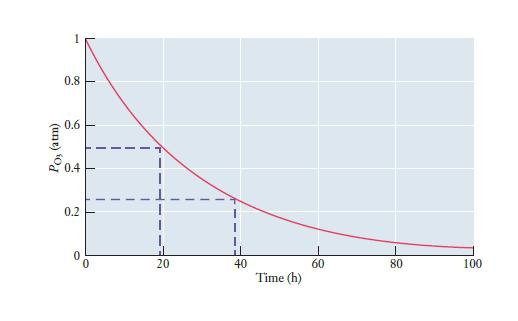
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





