The reaction shown below is involved in the refining of iron. (The table that follows provides all
Question:
The reaction shown below is involved in the refining of iron. (The table that follows provides all of the thermodynamic data you should need for this problem.)
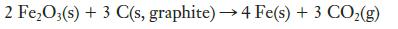
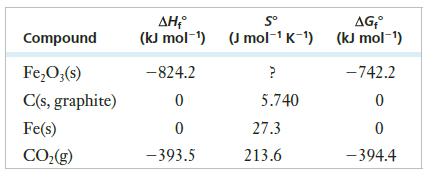
(a) Find ΔH° for the reaction.
(b) ΔS° for the reaction above is 557.98 J/K. Find ΔS° for Fe2O3(s).
(c) Calculate ΔG° for the reaction at the standard temperature of 298k. (There are two ways that you could do this.)
(d) At what temperatures would this reaction be spontaneous?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





