The table below presents measured rate constants for the reaction of NO with ozone at three temperatures.
Question:
The table below presents measured rate constants for the reaction of NO with ozone at three temperatures. From these data, determine the activation energy of the reaction in kJ/mol. (Assume the temperatures all have at least two significant figures.)
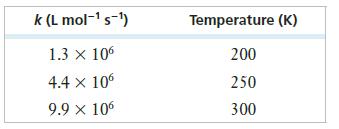
Transcribed Image Text:
k (L mol-¹ s-¹) 1.3 X 106 4.4 x 106 9.9 × 106 Temperature (K) 200 250 300
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
To determine the activation energy of the reaction in kJmol we can use the Arrhenius equation which ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Rate constants for a reaction were determined at five temperatures. From the following data, calculate the experimental energy of activation and then calculate G¡, H¡, and S¡ for...
-
Risk Assessment Homework In this assignment, you will perform a qualitative risk assessment, using a template that has been provided below. A listing of threats has been prepopulated for you. These...
-
The table below presents the scores of 10 states on each of six variables, which are three measures of criminal activity as dependent variables and three possible independent variables: unemployment...
-
?
-
On January 1, the company had 100,000 common shares outstanding. This same number of common shares was outstanding throughout the year. The company also had 30,000 shares of 5%, $100 par preferred...
-
Lilliput, a one-product mail-order firm, buys its product for $60 per unit and sells it for $130 per unit. The sales staff receives a 10% commission on the sale of each unit. Its December income...
-
Use information from Section 6.7 to estimate which form of electromagnetic radiation is the lowest energy ionizing radiation. Data from section 6.7 When we first introduced the concept of the...
-
The South Division of Wiig Company reported the following data for the current year. Sales .............. $3,000,000 Variable costs ........... 1,950,000 Controllable fixed costs ........ 600,000...
-
6. MAC =9 The use of energy is one of the major causes of pollution and greenhouse gases that lead to climate change. It is desirable to reduce the emissions from energy use. Suppose there are two...
-
The table below presents rate constants measured at three temperatures for the following reaction, which is involved in the production of nitrogen oxides in internal combustion engines. (Assume the...
-
The following rate constants were obtained in an experiment in which the decomposition of gaseous N 2 O 5 was studied as a function of temperature. The products were NO 2 and NO 3 . Determine E a for...
-
One of the statistics that the College Board monitors is the rising tuition at private and public four-year colleges. The tuition and fees for private and public four-year colleges for the 19801981...
-
A rock thrown with speed 1 2 . 0 m / s and launch angle 3 0 . 0 ( above the horizontal ) travels a horizontal distance of d = 1 7 . 5 m before hitting the ground. From what height was the rock...
-
"What is the role of strategic intent and visioning in setting aspirational goals, inspiring organizational commitment, and mobilizing resources towards achieving transformative outcomes?...
-
Mandated nurse-to-patient ratios affect potentially all hospitals and spark much debate in terms of their merits and feasibility of enforcement. The ratio itself is not precise and is influenced by...
-
(30 pts.) Given the four data points (-1,1), (0,1), (1,2),(2,0), determine the interpolating cubic polynomial using the monomial basis; using the Lagrange basis; using the Newton basis. Show that...
-
5. The sun has a radius of 7.0x105 km and spins (rotates) around its central axis with a period of 25 days. What would be the new period if the sun was to shrink (decrease in size), under the action...
-
Presented below are the comparative statements for Titan Company. The following additional information is provided: 1. In 2014, Titan decided to switch its depreciation method from the straight-line...
-
Differentiate the following terms/concepts: a. Personality types and money attitudes b. Planners and avoiders c. Moderating and adapting to biases d. "Perfectible judges" and "incorrigible judges"
-
For phenanthrene, the measured lifetime of the triplet state ( p ) is 3.3 s, the fluorescence quantum yield is 0.12, and the phosphorescence quantum yield is 0.13 in an alcoholether glass at 77 K....
-
In this problem you will investigate the parameters involved in a single-molecule fluorescence experiment. Specifically, the incident photon power needed to see a single molecule with a reasonable...
-
A central issue in the design of aircraft is improving the lift of aircraft wings. To assist in the design of more efficient wings, wind-tunnel tests are performed in which the pressures at various...
-
Do judges ever confer with law enforcement to assist in determining an appropriate sentence? Please explain
-
What does "the scope of the duty of disclosure is limited to what is reasonable in any given instance" mean?
-
The military's death penalty law requires court members (the military's equivalent to civilian jurors) to possess specific requirements, in addition to being the best qualified by reason of age,...

Study smarter with the SolutionInn App


