Two weak acids, A and B, are each titrated with the same solution of a strong base,
Question:
Two weak acids, A and B, are each titrated with the same solution of a strong base, producing the following titration curves.
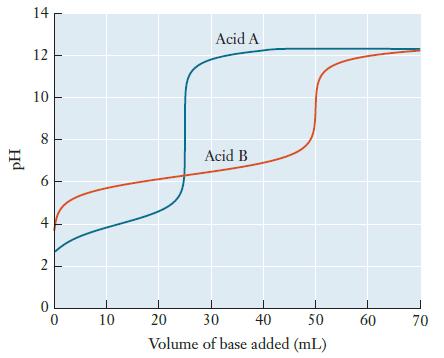
(a) Which acid has a larger Ka value, A or B? Explain.
(b) Use the titration curves to estimate the pH at the equivalence point for each acid. Explain why these values are not the same.
(c) If equal volumes of Acid A and Acid B were used, which one had the higher initial concentration? Explain.
Transcribed Image Text:
pH 14 12 10 8 6 4 2 0 10 1 20 Acid A Acid B I 50 30 40 Volume of base added (mL) 60 70
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a Halfway to the equivalence point the pH will be equa...View the full answer

Answered By

Livingstone Lowoi
Hi! My name is Livingstone. You can call me UnemployedProfessor. Originally I am from Africa, but I immigrated to the US and settled in Phoenix, AZ.
I am a professional academic writer with over five years of experience in writing custom essays, research papers, and thesis/dissertations. I value customer requirements by delivering top-notch essays that are plagiarism-free. Besides that, I always make sure that the composition adheres to the assignment details and instructions. As the client is in charge of their product, my job is to make sure that they sit back and give me the opportunity to prepare a custom-made and high-quality paper that will guarantee them an excellent grade.
I have handled assignments at various academic levels ranging from Undergraduate, Masters, and Doctorate levels. As a professional writer, I have majored in the following disciplines: English and Literature, Sociology, Nursing, Philosophy, Law, Social Sciences, Business Studies, Finance, Marketing, Management, Healthcare, Nutrition, Communication and Media, History, and Political Science. I have exceptional skills in project management tasks and the use of applications such as Excel, Word, PowerPoint, and Access.
My essay writing services are reputable thanks to an outstanding record in the quality and punctuality of the assigned papers. I am always available for my client; I pay close consideration to the various requirements regarding language and content. Consequently, I always ensure that the client is regularly updated with the writing process by providing drafts at each stage. I conduct extensive research for my custom writings to acquire information from different sources that would help produce a high-quality paper. I have the best Plagiarism Software and Scanners such as Turnitin, CopyScape, and Safeassign to ensure that the paper writing service is 100% original. I am well-conversant with all the appropriate referencing styles such as Harvard, APA, Chicago/Turabian, MLA, Oxford, Vancouver, etc.
Throughout my experience, I have acquired substantial academic writing skills and knowledge that has enabled me to complete assigned tasks with the highest level of accuracy, precision, and professionalism. As a result, I am always committed to ensuring that I diligently handle all papers professionally by addressing every bit of the instructions provided by the customer.
If you are seeking excellent grades in your assignments, don’t hesitate to order directly from me!
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Project the 2 4-1 IV design in Example 8-1 into two replicates of a 2 2 design in the factors A and B. Analyze the data and thaw conclusions. Example 8-1: Consider the filtration rate experiment in...
-
In this problem, you will use your knowledge of the phase plane and stability to analyze a simple model of glycolysis. The glycolysis pathway that first convert glucose into fructose-6-phosphate...
-
The overall goal of this problem is to compute the PV and PT equilibrium diagramsfor a single component fluid described by the van derWaals equation of state. Let us recall the key things we need to...
-
5 Question 42 (2.5 points) Consider a put option that gives the long position the right to sell the underlying asset for $12.34 in 5.67 years. The continuously compounded risk free rate of interest...
-
What three activities should you perform in Phase 1 of the writing process for a business letter?
-
Plasto Corporation manufactures a variety of plastic products including a series of molded chairs. The three models of molded chairs, which are all variations of the same design, are Standard (can be...
-
What do you like best about working at this company?
-
Krause Industries balance sheet at December 31, 2013, is presented below. Additional information accumulated for the budgeting process is as follows. Budgeted data for the year 2014 include the...
-
15. Jenny borrows $20,000 for her car at an interest rate of 2.5% to be paid off over five years, during which time the inflation rate averages 7%
-
The U.S. International Trade Commission's committee in charge of the global safeguard investigation involving imports of steel has announced its recommendations to be forwarded to the president. Of...
-
An electrolysis cell that deposits gold (from Au + ions) operates for 15.0 minutes at a current of 2.30 a. What mass of gold is deposited? Strategy As in any stoichiometry problem we need a balanced...
-
No e-mail messages are eloquent creations. Some love letters are eloquent creations. Therefore, some love letters are not e-mail messages. Determine whether the following arguments are best...
-
Bill, age 27, earns a salary of $100,000 in 2020 when the consumer price index was 258. Bill's father earned a salary of 30,000 when the consumer price index was 85.5. As for 2020, who earned a...
-
= 4. If a mass ma 2m has initial velocity 4 m/s and mass m m is initially at rest, they undergo perfectly inelastic collision. Calculate: (a) the final velocity and CACA OD COAST DIMAMYG (b) %...
-
"I had an empire of the sun. It was happy and large. White people took it away from me. Little charango. My defeated race cries, defeated by another civilization." What historical and political event...
-
Delbert makes homemade salsa and sells it at his roadside produce stand. Delbert's weekly cost for making salsa can be determined using the equa- tion C=2.75j+40, where C represents the cost in dol-...
-
If courts in the United States suddenly dispensed with informal models of criminal justice and instead relied on formal procedure, what do you think would happen to the judicial system? explain and...
-
For each of the following situations, indicate whether you agree or disagree with the financial reporting practice employed and state the basic assumption, constraint, or accounting principle that is...
-
Does log 81 (2401) = log 3 (7)? Verify the claim algebraically.
-
The shaft is made of L2 tool steel, has a diameter of 40 mm, and is fixed at its ends A and B. If it is subjected to the couple, determine the maximum shear stress in regions AC and CB. 2 kN 2 kN 50...
-
The Am1004-T61 magnesium tube is bonded to the A-36 steel rod. If the allowable shear stresses for the magnesium and steel are (Ï allow ) mg = 45 MPa and (Ï allow ) st = 75 MPa,...
-
The Am1004-T61 magnesium tube is bonded to the A-36 steel rod. If a torque of T = 5 kN m is applied to end A, determine the maximum shear stress in each material. Sketch the shear stress...
-
2. Consider the ammonia synthesis reaction N2(g) + 3H2(g) 2NH3(g). A mixture of N and H2 at stoichiometric molar ratio (i.e., 1:3) are placed in a catalytic reactor at T = 400 K and p = 2 bar. Find...
-
1. (19 points) An adsorption study was conducted by adding varying amounts of activated carbon to a series of air samples consisting of 1 L of air containing 10mL of a volatile gas. Different amounts...
-
1. Consider a liquid solution of species A and B at equilibrium with its vapor phase. The vapor is an ideal-gas mixture. The liquid phase, however, is non-ideal. The convention I activity coefficient...

Study smarter with the SolutionInn App


