Using the equations determine the equilibrium constant for the following reaction: HASO4 (aq) AsO4 (aq) + H+
Question:
Using the equations
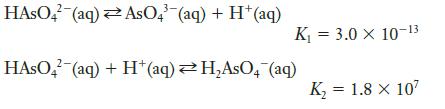
determine the equilibrium constant for the following reaction:
![]()
Transcribed Image Text:
HASO4 (aq) AsO4 (aq) + H+ (aq) K₁ = 3.0 X 10-13 HASO4 (aq) + H+ (aq)H₂AsO4 (aq) K₂ = 1.8 x 10²
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To determine the equilibrium constant for the reaction HAsO4aq AsO4aq 2H...View the full answer

Answered By

Pranav Makode
I am a bachelor students studying at professor ram meghe institute of technology and research. I have a great experience of being an expert. I have worked as an expert at helloexperts and solvelancer as a part time job. I have also worked as a doubt solver at ICAD SCHOOL OF LEARNING, which is in Amravati city. I have also worked as an Freelancer.
I have great experience of helping students, as described above. I can help any students in a most simple and understandable way. I will not give you have any chance for complaint. You will be greatfull to accept me as an expert.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
The equilibrium constant for the reaction SO3 SO2 + O has the following values: Determine the average heat of dissociation using graphical method. T 800 K 900 K 1000 K 1105K | 0.0319 | 0.153 | 0.540...
-
The following reaction has an equilibrium constant Kc equal to 3.59 at 900oC. For each of the following compositions, decide whether the reaction mixture is at equilibrium. If it is not, decide which...
-
The equilibrium constant for the following reaction is 1.0 Ã 10-3: Cr'. (aq) + H2EDTA2-(aq)--CrEDT A-(aq) + 2H' (aq) CH2-CO2 02GH CH EDTA N-CH,--CH2- O2C-CH2 CHy-CO2-...
-
The combined sewer system in city ABC is comprised of two parallel interceptors referred to as "North" and "South" lines. The southern line is connected to a newly built wastewater treatment plant....
-
Describe the types of cost-based pricing and the methods of implementing each.
-
Mountain Maples is a mail-order nursery dedicated to growing, selling, and shipping beautiful Japanese Maple trees. Located on a ridge-top in Mendocino County, Northern California, Mountain Maples...
-
Heritage, a general contractor, had filed a breach of contract action against an electrical subcontracting firm that had withdrawn its bid right before the contract was to be performed. A jury had...
-
Capitalization of Interest On December 31, 2009, Hurston Inc. borrowed $3,000,000 at 12% payable annually to finance the construction of a new building. In 2010, the company made the following...
-
On January 1, 2024, the general ledger of Dynamite Fireworks includes the following account balances: Accounts Cash Debit $24,900 Credit Accounts Receivable 6,300 Supplies 4,200 Land 61,000 Accounts...
-
For each of the following equations, write the equilibrium expression for the reverse reaction. (a) 2 C(s) + O 2 (g) 2 CO(g) (b) AgCl(s) Ag + (aq) + Cl (aq)
-
For the equilibria given below, determine each of the following: (a) Equilibrium expressions for K 1 and K 2 (b) The equation for the reaction that is the sum of the two equations (c) The...
-
Suppose you decided to start a new fast food restaurant business to compete with McDonalds. REQUIRED A. Describe your organizational vision and list two core competencies. B. Describe your general...
-
2. www.rwcruises.com is the official website for RW Cruise Packages. a. Besides offering service to book cruise package online, propose THREE (3) other functions of www.rwcruises.com (6 marks) b....
-
Select all that apply In variance analysis, measuring accomplishments centers on two key computations. Identify the two computations.
-
a Quality engineer wanted to construct and s - chart control process variability. The average standard deviation of the collected sample was 0 . 2 . Samples with 1 5 components for each determine the...
-
P9.3.1 Determine what the following pseudo-Java method outputs on input n. Prove your answer by induction on the call tree: integer foo (natural n) { if (n
-
A taxpayer owns an interest in a partnership which rents property. This is his sole source of income and produces defined taxable income. Together, he receives $70,000 of rental income. One of the...
-
The table lists the maximum and minimum hole and shaft dimensions for a variety of standard press and shrink fits. The materials are both hot-rolled steel. Find the maximum and minimum values of the...
-
Identify the most stable compound:
-
The shaft is supported by a smooth thrust bearing at A and a smooth journal bearing at B. Determine the resultant internal loadings acting on the cross section at C. 600 N/m -1 m--1 m---1 m--1.5...
-
Determine the resultant internal loadings acting on section bb through the centroid C on the beam. B' 900 Ib/ft 60 3 ft 30 A 6 ft
-
Determine the resultant internal normal and shear force in the member at (a) section aa and (b) section bb, each of which passes through the centroid A. The 500-lb load is applied along the...
-
Populate the table below with respect to important key production variables Marginal Physical Product (MPP), Average Physical Product (APP), Daily Labor Use (L) Daily Output Level (Q) AQ/AL Q/L 10...
-
How to calculate the accumlated depreciation buildings number on a balance sheet?
-
What is the narrative in QuickBooks for an account with the account type of expenses and detail type of advertising promotional?

Study smarter with the SolutionInn App


