Which of the following compounds would you predict to be soluble in water at room temperature? (a)
Question:
Which of the following compounds would you predict to be soluble in water at room temperature?
(a) KClO3,
(b) CaCO3,
(c) BaSO4,
(d) KMnO4
Strategy Solubility guidelines for common ions are given in Table 3.1. So we will identify the ions in each compound and consult the table as needed to determine the solubilities.
Table 3.1
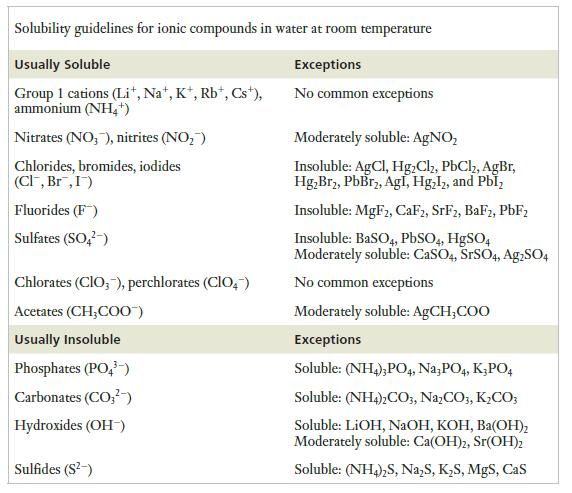
Transcribed Image Text:
Solubility guidelines for ionic compounds in water at room temperature Usually Soluble Exceptions No common exceptions Group 1 cations (Li+, Na+, K+, Rb+, Cst), ammonium (NH4+) Nitrates (NO3), nitrites (NO₂) Chlorides, bromides, iodides (Cl, Br, I) Fluorides (F) Sulfates (SO²) Chlorates (ClO3), perchlorates (CIO) Acetates (CH₂COO) Usually Insoluble Phosphates (PO4³-) Carbonates (CO²) Hydroxides (OH-) Sulfides (S²-) Moderately soluble: AgNO₂ Insoluble: AgCl, Hg2Cl2, PbCl2, AgBr, Hg₂Br₂, PbBr₂, Agl, Hg₂12, and Pbl₂ Insoluble: MgF2, CaF2, SrF2, BaF2, PbF₂ Insoluble: BaSO4, PbSO4, HgSO4 Moderately soluble: CaSO4, SrSO4, Ag2SO4 No common exceptions Moderately soluble: AgCH,COO Exceptions Soluble: (NH4), PO4, Na3PO4, K3PO4 Soluble: (NH4)2CO3, Na₂CO3,K₂CO3 Soluble: LiOH, NaOH, KOH, Ba(OH)₂ Moderately soluble: Ca(OH)2, Sr(OH)2 Soluble: (NH4)₂S, Na₂S, K₂S, MgS, CaS
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a KClO 3 is potassium chlorate From the solubility guidelines in Table 31 we see that compound containing K and ClO 3 tend to be soluble and that no c...View the full answer

Answered By

Sinmon Warui Kamau
After moving up and down looking for a job, a friend introduced me to freelance writing. I started with content writing and later navigated to academic writing. I love writing because apart from making a living out of it, it is also a method of learning and helping others to learn.
5.00+
40+ Reviews
45+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Which of the following compounds would you expect to be the most generally reactive, and why? DO
-
Which of the following compounds would you expect to be most acidic? Justify your choice.
-
Which of the following compounds would you expect to be more reactive in an SN2 reaction? CH3 CH3Br CH3H
-
You have a net income of $40 000 per year. Your expenses include the following: Rent: $800 per month Insurance: $225 per semi-annually Car Payment: $315 per month Car Expenses: $1 000 per year ...
-
Marian Kirk wishes to select the better of two 10-year annuities, C and D. Annuity C is an ordinary annuity of $2,500 per year for 10 years. Annuity D is an annuity due of $2,200 per year for 10...
-
The shares of a firm trade on the stock market at a total of $1.2 billion and its debt trades at $600 million. What is the value of the firm (its enterprise value)?
-
Plaintiffs James and Betty Tonkovich own approximately 850 acres of in Belmont County, Ohio. Plaintiffs belong to a group of landowners known as Belmont Leasing Group, which leases land for oil and...
-
Forest City has recently implemented GAAP reporting and is attempting to determine which of the following special revenue funds should be classified as major funds and therefore be reported in...
-
You need your client to send you a copy of a paper receipt so that you can match it to a gas expense in their QuickBooks Online. You create a client request and add a screenshot of the expense entry...
-
Propane, C 3 H 8 , is used as a fuel in many applications, including gas barbecue grills. Because of its widespread use, extensive research is underway to develop ways to produce propane from...
-
Name each of the following compounds: (a) MgCl 2 , (b) Fe (NO 3 ) 2 , (c) Na 2 SO 4 , (d) Ca (OH) 2 , (e) FeSO 4
-
Compute the derivative of the following functions. g(x) = x/e 3x
-
What is the difference between a yeast and a mold growth form? Provide an example of a beneficial fungus and one that is associated with human disease. What does it mean that fungi are "opportunists"?
-
If there is heavy smoke or extreme heat in a compartment that is being searched, rescuers should: Select one: a. move below the smoke level. b. attempt to extinguish the fire. c. stand to get a...
-
Vector A has magnitude 16.0 m and vector B has magnitude 13.0 m. The scalar product A - B is 105 m. What is the magnitude of the vector product between these two vectors? Express your answer with the...
-
A student has been asked to dilute a PCR DNA sample to 40 ng L 1. Knowing that the concentration of the stock solution of the PCR DNA sample is 180 ng uL and its volume is 50 L, how much dHO should...
-
Which piece of equipment is used to quickly drain water from the LDH hose before it is stored? Select one: a. Hose bridge b. LDH drainage hose roller c. Chafing block d. LDH drainage spout
-
Charlie is married and files a joint return. He reports the following items of income and loss for the year: Salary .................. $ 120,000 Activity A (passive) ............. 13,000 Activity B...
-
The production budget of Artest Company calls for 80,000 units to be produced. If it takes 30 minutes to make one unit and the direct labor rate is $16 per hour, what is the total budgeted direct...
-
In the reversible adiabatic expansion of 1.75 mol of an ideal gas from an initial temperature of 27.0C, the work done on the surroundings is 1300. J. If C V ,m = 3/2R, calculate q, w, U, and H.
-
For a given set of conditions, the fugacity of a gas is greater than the pressure. What does this tell you about the interaction between the molecules of the gas?
-
Predict the major product of the reaction between 1-butanol and: (a) PBr 3 (b) SOCl 2 , py (c) HCl, ZnCl 2 (d) H 2 SO 4 , heat (e) PCC, CH 2 Cl 2 (f ) Na 2 Cr 2 O 7 , H 2 SO 4 , H 2 O (g) Li (h) NaH...
-
9. Determine the scale factor that was used to transform diagram X into diagram Y. Express your scale factor to one decimal place. (1mark) X 2.0 cm 7.0 cm Y 7.0 cm 24.5 cm 3 10. Given the linear...
-
Required: 1. Create a new worksheet "Chapter5 EX3", and enter the previous data in it. 2. Insert necessary equations or functions in the related cells to calculate total current assets, total assets,...
-
lem: Let a > 1, and g(a) is a function of a Consider the following maximization prob- = max f(x; a) a logr + (1a)x +9(a) ZER 1. Write down the first order conditions for this problem with respect to...

Study smarter with the SolutionInn App


