A 1:1 mole ratio of CO (g) and H 2 (g) is called water gas.
Question:
A 1:1 mole ratio of CO (g) and H2 (g) is called water gas. It is used as a fuel because it can be burned in air: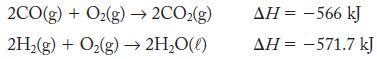
(a) Find the number of moles of CO (g) and H2 (g) Present in 10.0 g water gas. (Remember that they are present in a 1:1 mole ratio.)
(b) Use the preceding thermochemical equations to fi nd the enthalpy change when 10.0 g water gas is burned in air.
Transcribed Image Text:
2CO(g) +O₂(g)→ 2CO₂(g) 2H₂(g) + O₂(g) → 2H₂O(l) AH = -566 kJ AH = -571.7 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a To find the number of moles of CO g and H2 g present in 100 g of water gas we need to use their mo...View the full answer

Answered By

Rubina Kousar
I had done Msc Cs And doing a job at College Level I am very kind hearted to my students my method of teaching is verly good Not so strict My Teaching experience is very good Students are So obedient also i taught them in very pleasent environmnent.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
In a hydrogen fuel cell, hydrogen and oxygen gases react to produce water vapour. What volume of hydrogen at 40C and 150 kPa can be burned in a fuel cell using 300L of oxygen gas measured under the...
-
Read the case study "Southwest Airlines," found in Part 2 of your textbook. Review the "Guide to Case Analysis" found on pp. CA1 - CA11 of your textbook. (This guide follows the last case in the...
-
9. A molybdenum-vanadium alloy of composition 50wt%Mo - 50wt%V is slowly cooled from a temperature of 2600C to 1800C. Determine: a) At what temperature does the first solid phase form? b) What is the...
-
The Kelvin equation, (17-16), predicts that solubility increases to infinity as the crystal diameter decreases to zero. However, measurements by L. Harbury [J. Phys. Chem., 50, 190-199 (1946)] for...
-
Gives the first derivative of a function y = (x). (a) At what points, if any, does the graph of have a local maximum, local minimum, or inflection point? (b) Sketch the general shape of the graph. y...
-
On 1 October 2025, Kevin Lindsay and Sharon Foster formed a partnership. Some business assets and the liabilities of Lindsay were assumed by the partnership, and these are listed below at both...
-
Slaton Corporation traded a used truck for a new truck. The used truck cost $20,000 and has accumulated depreciation of $17,000. The new truck is worth $35,000. Slaton also made cash payment of...
-
15 16 17 18 In 1987, an agreement was formulated by the United Nations Environment Programme (UNEP) to freeze the production of "X" to prevent depletion of "Y". "X" and "Y" respectively referred here...
-
Light-It-Up Company maintains and repairs warning lights, such as those found on radio towers and lighthouses. Light-It-Up Company prepared the following end-of-period spreadsheet at August 31, 20Y5,...
-
What mass of ethylene, C 2 H 4 (g), must be burned to produce 3420 kJ of heat, given that its enthalpy of combustion is -1410.1 kJ/mol?
-
When a 2.30-g sample of magnesium dissolves in dilute hydrochloric acid, 16.25 kJ of heat is released. Determine the enthalpy change for the thermochemical equation Mg(s) + 2HCl(aq) MgCl(aq) + H2(g)...
-
The displayed homomorphism condition for an isomorphism in Definition 3. 7 is sometimes summarized by saying," must commute with the binary operation(s)." Explain how that condition can be viewed in...
-
In Problems 4-13, decide whether the statement is true or false. If it is false, tell what is wrong. If row 7 of a matrix \([B]\) is multiplied by -2 and then added to row 6 of \([\mathrm{B}]\), then...
-
@NicoleD to @Whole Foods Market: Did you guys discontinue the heavenly super stars gummies ?!?!?! Prepare a response explaining that you had to check with WFMs private label team to see whether this...
-
Suppose you are tossing a fair coin 20 times. What is the probability that you will toss exactly five heads? What is the probability that you will toss five or fewer heads?
-
A system in unity feedback configuration has the loop transmittance \[ \mathrm{G}(s)=\frac{\mathrm{K}}{s(2 s+1)} \] Using frequency response techniques, do the following: (a) Find \(\mathrm{K}\) to...
-
What do you think Eminems incentive is to give a free show? Was his decision made in self-interest or in the social interest? Explain your answer. Hundreds line up for 5 p.m. Eminem ticket giveaway...
-
a. What is the payback period on each of the following projects? b. Given that you wish to use the payback rule with a cutoff period of two years, which projects would you accept? c. If you use a...
-
What are the main distinctions between the different schools of legal interpretation?
-
Describe what you would observe if you heated the liquid mixture at the composition corresponding to point i in Figure 9.24b from a temperature below T a to 118°C. Figure 9.24b 118 Vapor 100 Tj...
-
The heat of fusion of water is 6.008 10 3 J mol 1 at its normal melting point of 273.15 K. Calculate the freezing point depression constant K f .
-
At 39.9C, a solution of ethanol (x 1 = 0.9006, P * 1 = 130.4 Torr) and isooctane (P * 2 = 43.9 Torr) forms a vapor phase with y 1 = 0.6667 at a total pressure of 185.9 Torr. a. Calculate the activity...
-
The foundation for a cylindrical water tank is a cylinder 27 ft in diameter and 3 ft high. How many cubic ft of concrete are needed to build the foundation?
-
Exercise 8 - Calculating and Comparing Return on Invested Capital (ROIC) Apple v. Blackberry Return on Invested Capital (ROIC) is a profitability ratio that measures how effective the firm is at...
-
This week our focus is on leader-follower relationships. For this week's discussion, consider the practices, policies, and norms you would expect to find in an organization that prides itself on...

Study smarter with the SolutionInn App


