Assuming that each reaction is elementary, predict the rate law and molecularity. (a) NO(g) + NO3(g) 2NO(g)
Question:
Assuming that each reaction is elementary, predict the rate law and molecularity.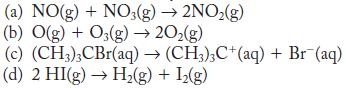
Transcribed Image Text:
(a) NO(g) + NO3(g) 2NO(g) (b) O(g) + O3(g) 20(g) (c) (CH3)3CBr(aq) (CH3)3C+ (aq) + Br (aq) (d) 2 HI(g) H(g) + 1(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
In chemical kinetics the rate law expresses the relationship between the rate of a chemical reaction ...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Write the expected rate law and molecularity for each of the following elementary reactions in the gas phase. Strategy The rate law for an elementary reaction is derived directly from its...
-
Write the rate law and the molecularity for each of the following elementary reactions. (a) NO+NOCl NO + NOCI (b) NO + SO NO + SO3 (c) NO4 2NO
-
Write the rate law and the molecularity for each of the following elementary reactions. (a) CH5C1 CH4 + HC1 (b) NO + O3 NO + O (c) HI + CHI CH6 + 1
-
a. Distinguish between internal common equity and new common stock. b. Why is there a cost associated with internal common equity? c. Describe two approaches that could be used in computing the cost...
-
Find Vad in the network infigure. 3V +) 12 V 4 V(+ e + 3V
-
How does the auditors control risk assessment affect the preliminary audit program?
-
Use the all-possible-regressions selection on the wine quality of young red wines data in Table B.19. Perform a thorough analysis of the best candidate models. Compare your results with stepwise...
-
A particle moves in a spherically symmetric force field with potential energy given by U(r) = k/r. Calculate the Hamiltonian function in spherical coordinates, and obtain the canonical equations of...
-
A simple loan with a present value of $5500 will be paid off in 5 years. If the interest rate is 7% what will the future payment be?
-
Evaluate each of the following proposed mechanisms to determine whether it is consistent with the experimentally determined stochiometry and rate law, and identify intermediates, if any. 2NO2+O3 NO5...
-
Nitrogen dioxide can react with ozone to form dinitrogen pentoxide and oxygen. A two-step mechanism has been proposed. Identify the rate-limiting step. 2NO(g) + O3(g) NO5(g) + O(g) rate = /[NO][03]
-
Wal-Mart, Inc., has net income of $9,054,000 on net sales of $256,329,812. The company has total assets of $104,912,112 and stockholders equity of $43,623,445. Use the extended DuPont identity to...
-
What are the benefits and risks of an integrating social and environmental data into annual financial reports, versus a separate report for Social and Environmental issues?
-
How can mindfulness, along with neuroscience/neuroleadership help a leader focus his or her followers? Please not that you do not have to cite the articles or include the references. It should...
-
Employers use the various types of social media in the workplace, especially for recruiting purposes. The increasing use of social media in the 21st century workplace can present issues for a...
-
Discuss about the qualities of a good and bad leader. What are the top 3 qualities you believe are important in effective leaders and why? Through experience and what you have seen, what are 2-3...
-
Consider differences among PMOs, portfolios, and programs and how an organization manages the project identification and selection process. Share how a company or a familiar company organizes its...
-
Unisonic Company had sales recenues for the year of $1,750. Average working capital; property, plant, and equipment; and shareholders' equity were $250, $525, and $1,500, respectively. (All figures...
-
X-1 Find the domain of the function f(x) : x 1 2 - O (-00, -1) U (-1, ) O (-00, 1) U (1, ) O -00, -1) U (-1, 1) U (1, 0) O (- 1, 1)
-
Determine the vertical displacement of joint B. For each member A = 400 mm 2 , E = 200 GPa. Using Castiglianos theorem. 1.5 m A JO 45 kN 2 m 2 m
-
Determine the vertical displacement of joint B. For each member A = 400 mm 2 , E = 200 GPa. Use the method of virtual work. 1.5 m A JO 45 kN 2 m 2 m
-
Determine the vertical displacement of joint A. Each bar is made of steel and has a cross-sectional area of 600 mm 2 . Take E = 200 GPa. Using Castiglianos theorem. B. 2 m Abc 1.5 m 1.5 m 5 kN
-
2. Write a Java program that generates StringIndexOutOfBoundsException exception. b. Ensure that the exception is handled by using the appropriate exception type. 3. Explain the difference between...
-
public class Test{ public double avgFirstN(int N) { double sum = 0; if (N
-
What does cultural dexterity refer to in an organization? Explain

Study smarter with the SolutionInn App


