Nitrogen dioxide can react with ozone to form dinitrogen pentoxide and oxygen. A two-step mechanism has been
Question:
Nitrogen dioxide can react with ozone to form dinitrogen pentoxide and oxygen.![2NO(g) + O3(g) NO5(g) + O(g) rate = /[NO][03]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/3/5/5/041659664e176f1c1704355041072.jpg)
A two-step mechanism has been proposed. Identify the rate-limiting step.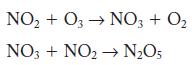
Transcribed Image Text:
2NO(g) + O3(g) NO5(g) + O(g) rate = /[NO][03]
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
To identify the ratelimiting step in the twostep reaction mechanism we look at each step and compare ...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
The following mechanism has been proposed to account for the rate law of the decomposition of ozone to O2(g): Apply the steady-state hypothesis to the concentration of atomic oxygen, and derive the...
-
The following mechanism has been proposed for the reaction of NO with H2 to form N2O and H2O: NO(g) + NO(g) N2O2(g) N2O2 + H2(g) N2O(g) + H2O(g) (a) Show that the elementary reactions of the...
-
The following mechanism has been proposed for the gas-phase reaction of H2 with ICl: H2(g) + ICl(g) HI(g) + HCl(g) HI(g) + ICl(g) I2(g) + HCl(g) (a) Write the balanced equation for the overall...
-
As an employer, what are steps you can take to avoid discrimination litigation?
-
Find Vbd in the circuit infigure. 4 V 6V 12 V
-
Categorize the eleven points of vulnerability to misstatement errors related to manual input, computer processing, and error correction activities in a computerized information system.
-
Use the all-possible-regressions selection on the fuel consumption data in Table B.18. Perform a thorough analysis of the best candidate models. Compare your results with stepwise regression....
-
Online Products is considering adopting the balanced scorecard and has compiled the following list of possible performance measures. Select the balanced scorecard perspective that best matches each...
-
An investment portfolio began at $1,000,000, but has a current value of $782,655.71 with a standard deviation of 5 and a risk free ratio of 3.13%. 1. Calculate the Sharpe and Information Ratios and...
-
Assuming that each reaction is elementary, predict the rate law and molecularity. (a) NO(g) + NO3(g) 2NO(g) (b) O(g) + O3(g) 20(g) (c) (CH3)3CBr(aq) (CH3)3C+ (aq) + Br (aq) (d) 2 HI(g) H(g) + 1(g)
-
The gas-phase reaction of nitrogen monoxide with chlorine proceeds to form nitrosyl chloride. 2NO(g) + Cl(g) 2NOC1(g) rate = k[NO][C1] Evaluate the following proposed mechanism to determine whether...
-
Use the shifting Theorem to determine where f(t) = [e-(t-2) e-2]u(t-2)
-
Describe how to integrate the visual tool you found it into a stakeholder analysis. Also, show how to create a template of stakeholder analysis for a project including an example of the visual tool...
-
At its core, lean deals with the reduction of waste. In the OM context, we typically attempt to reduce inventories, waste from production, and other types of waste more generally. Respond to the...
-
1. What would be the key logistics activities for Ford that would allow it to improve its situation? Describe them 2. What are the decision factors that would allow the distribution process to be...
-
1. How are project management and consulting engagement management the same or different? In two paragraphs or less, please describe two ways they are similar and two ways they are different. 2. In...
-
Research an organization that practices Global Sourcing. Explain if the organization is successful What are the Global Sourcing best practices based on your research? provide details for your...
-
Ralite Company had net income for the year of $20 Million. It had 2 Million sharees of comon stock outstanding, with a year-end market price of $82 a share. Dividends during the year were $5.74 a...
-
In July 2013, cnet.com listed the battery life (in hours) and luminous intensity (i. e., screen brightness, in cd/m2) for a sample of tablet computers. We want to know if screen brightness is...
-
Determine the vertical displacement of joint A. Each bar is made of steel and has a cross-sectional area of 600 mm 2 . Take E = 200 GPa. Use the method of virtual work. B. 2 m AGe 1.5 m 1.5 m 5 kN
-
Use the Muller-Breslau principle to sketch the general shape of the influence line for (a) The moment at A and (b) The shear at B. A
-
Use the Muller-Breslau principle to sketch the general shape of the influence line for (a) The moment at A and (b) The shear at B. TTI A
-
Assume that a program runs in f(n) microseconds, where f(n) is the function given on the left. Fill in how large an input n can be calculated, given each of the runtimes given along the top. I have...
-
For audiometric equipment, what is the meaning of blue-colored or blue-featured equipment?
-
What are the `deterrent' and `incapacitation' effects in the Becker model of crime? What are the main findings from academic literature about these factors with relevant empirical evidence, the...

Study smarter with the SolutionInn App


