Calculate the hydroxide ion concentration, the pOH, and the pH of the solution made when 1.00 g
Question:
Calculate the hydroxide ion concentration, the pOH, and the pH of the solution made when 1.00 g barium hydroxide dissolves in enough water to produce 500.0 mL of solution.
Strategy
First, calculate the amount of barium hydroxide from the mass and molar mass. Next, calculate the amount of hydroxide ion from the coefficients of the chemical equation; then calculate the concentration and pH from the amount of hydroxide ion and the volume of the solution.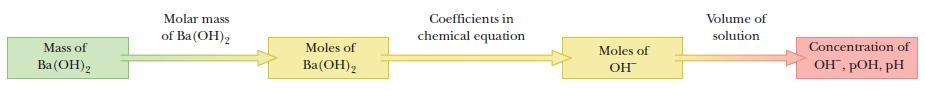
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
Amount of BaOH2 100 g BaOH2 X 1 mol BaOH2 1713 g BaOH2 584 x 103 mol BaOH2 Use the chemical equ...View the full answer

Answered By

Ajeet Singh
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions.
I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life.
I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge.
I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields.
Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a teacher. I have done plenty of studying and lots of learning on many exciting and challenging topics. All these experiences have influenced my decision to take on the teaching role in various capacities. As a tutor, I am looking forward to getting to understand your needs and helping you achieve your academic goals. I'm highly flexible and contactable. I am available to work on short notice since I only prefer to work with very small and select groups of students.
4.90+
7+ Reviews
15+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Soluble nontoxic salts such as Fe 2 (SO 4 ) 3 are often used during water purification to remove soluble toxic contaminants, because they form gelatinous hydroxides that encapsulate the contaminants...
-
QUESTION 1 When propane undergoes complete combustion, the products are carbon dioxide and water.? ? ? ? __ C 3 H 8 (g) + __ O 2 (g) ? __ CO 2 (g) + __ H 2 O(g)What are the respective coefficients...
-
Methylamine, CH 3 NH 2 , is a weak base used as a building block for some pharmaceuticals. If you prepare an aqueous solution of methylamine for use in a synthesis, you might need to know its pH to...
-
Maicom Construction Materials Inc. , hereinafter referred to as "MCM", is a construction materials company established in Moncton, New Brunswick. Its facilities (warehouse, store and offices) are...
-
How could you use 1H NMR to distinguish between the following pairs ofisomers? (a) CH3CH=CHCH2CH3 and CH2 H H2H () CH20CH2CH and CH3OCH2CH2CH3 (c) CHC,H and CH3CH2CH3 (d) HCICH)H and CH3CH=CHCH3
-
Smyth Company is acquired by Radar Corporation on July 1, 2011. Radar exchanges 60,000 shares of its $5 par stock, with a fair value of $20 per share, for the net assets of Smyth Company. Radar...
-
Consider the plot of power as a function of effect size for the two-sample t-test shown in Figure 4.3. a. Create a plot to show power as a function of both effect size and sample size while keeping...
-
During 2015, Walnut Company completed the following two transactions. The annual accounting period ends December 31. a. Paid and recorded wages of $130,000 during 2015; however, at the end of...
-
a. Discuss the role of Bank Supervision. b. Outline the selected Core Principles for Effective Banking Supervision. c. Discuss how to assess the effectiveness of supervision d. Evaluate the...
-
Calculate the hydrogen ion concentration and pH of the following: (a) 0.010 M HNO 3 (b) A solution prepared by diluting 10.0 mL of 0.50 M HClO 4 to 50.0 mL (c) A solution prepared by adding 9.66 g...
-
Picric acid, a weak acid, is dissolved in water to prepare a 0.100 M solution. Conductivity measurements at a particular temperature indicate that the picric acid is 82% ionized. Calculate K a and p...
-
Using the data from Problem 15, calculate the variance and standard deviation of the three investments, stock, corporate bond, and government bond. If the estimates for both the probabilities of the...
-
Burien Family Market has two family-owned grocery stores that have been in business for years. Whole Foods decides that Burien Family Market would be an excellent place to build one of its...
-
Patrick just finished designing a process improvement plan. To ensure that it is going to be successful, he embedded a step in the plan for a designated staff member to periodically verify that the...
-
How can the service concept be used to assess implications of design changes? How can the service concept be used to develop new and innovative concepts for the organization? Name the service concept...
-
Advise Cassie 4. "The rules relating to establishing a common intention constructive trust of the family home generate both uncertainty and unfairness." 1000 world article with example with case law
-
Summary of Readings Chapter 3: The Social Responsibility of Business Is to Increase Profits ( Friedman, 2009) what is his view on the role/purpose of business? what are some of his assumptions? what...
-
The case extensively discusses the idea of virtualization and the role it plays in the merger process. Go online to research this concept and prepare a report about what it entails, how it works,...
-
On the basis of the details of the following fixed asset account, indicate the items to be reported on the statement of cashflows: ACCOUNT Land ACCOUNT NO. Balance Date Item Debit Credit Debit Credit...
-
On average, the net electromagnetic power radiated by the Sun, its so-called luminosity (L), is 3.9 10 26 W. Determine the mean amplitude of the electric field due to all the radiant energy arriving...
-
Pulses of UV lasting 2.00 ns each are emitted from a laser that has a beam of diameter 2.5 mm. Given that each burst carries an energy of 6.0 J, (a) determine the length in space of each wavetrain....
-
A laser provides pulses of EM-radiation in vacuum lasting 10 -12 s. If the radiant flux density is 10 20 W/m 2 , determine the amplitude of the electric field of the beam.
-
What are some of the pros and cons of shifting elements of the portfolio, including rebalancing or making investment changes in terms of their tax liability? Showing full detail and specific examples...
-
Frank's Fluids has a market value of Debt of $5,000,000 and outstanding Equity Valued at $50,000,000. The company has a WACC of 10%. The company's NOPAT is $7,500,000 and its EBITDA is $8,200,000....
-
How are annuities used as a retirement strategy?what would be a specific example.What are some disadvantages of using an annuity as a part of your strategy?

Study smarter with the SolutionInn App


