Oxalic acid can decompose to formic acid and carbon dioxide: A graph of the concentration of oxalic
Question:
Oxalic acid can decompose to formic acid and carbon dioxide:![]()
A graph of the concentration of oxalic acid as a function of time follows.
(a) Write an expression for the rate of reaction in terms of a changing concentration.
(b) Calculate the average rate of reaction between 10 and 30 seconds.
(c) Calculate the instantaneous rate of reaction after 20 seconds.
(d) Calculate the initial rate of reaction.
(e) Calculate the instantaneous rate of formation of CO2 40 seconds after the start of the reaction.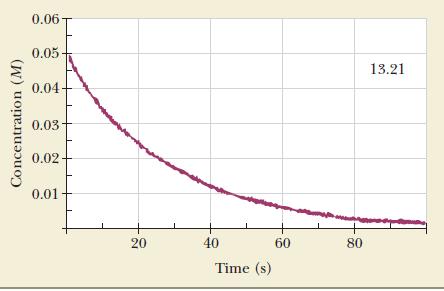
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





