Rank the following bases in order of relative strength, from weakest to strongest: formate ion, cyanide ion,
Question:
Rank the following bases in order of relative strength, from weakest to strongest: formate ion, cyanide ion, and acetate ion.
Strategy
This problem can be solved without calculations, because Table 15.6 lists values of Ka for each acid. The acids can be ranked in order of Ka, and the conjugate bases will be in the opposite order, because the stronger an acid, the weaker its conjugate base.
Table 15.6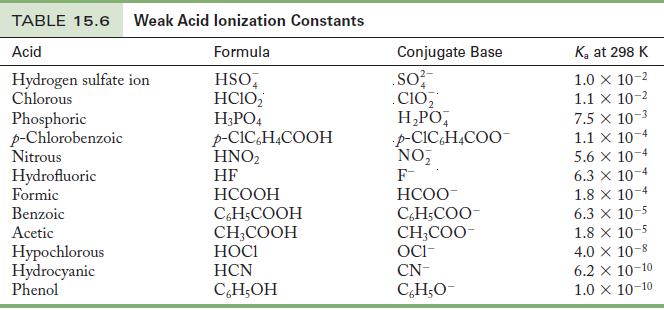
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





