The most intense peak in a mass spectrum is assigned a height of 100 units. The following
Question:
The most intense peak in a mass spectrum is assigned a height of 100 units. The following spectrum was obtained from a sample of an element. Use the data to calculate the atomic mass of the element. Identify the element.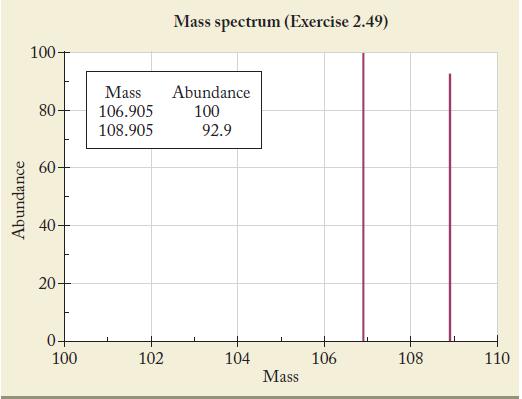
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





