Use the solubility product constant from Appendix F to determine whether a precipitate will form if 20.0
Question:
Use the solubility product constant from Appendix F to determine whether a precipitate will form if 20.0 mL of 1.0 × 10-6 M magnesium chloride is added to 80.0 mL of 1.0 × 10-6 M potassium fluoride.
Appendix F

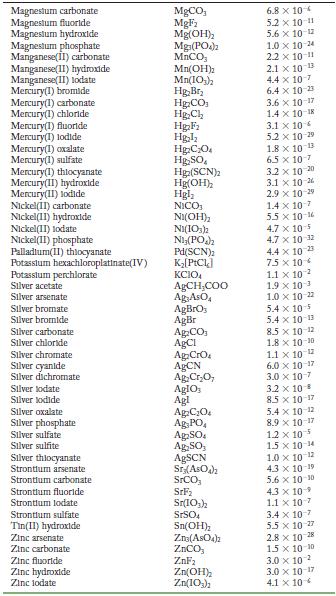
Transcribed Image Text:
Solubility Product Constants at 25 C Substance Aluminum phosphate Bartum carbonate Bartum chromate Barlum fluoride Barlum lodate Bartum phosphate Bartum sulfate Bismuth arsenate Cadmium arsenate Cadmium carbonate Cadmium cyanide Cadmium fluoride Cadmium hydroxide Cadmium lodate Cadmium phosphate Calcium carbonate Calclum fluoride Calcium hydroxide Calcium lodate Calcium phosphate Calcium sulfate Cerlum(III) lodate Cobalt(II) arsenate Cobalt(II) phosphate Copper(1) bromide Copper(1) chloride Copper(1) todide Copper(1) thiocyanate Copper(11) arsenate Copper(II) hydroxide Copper(II) lodate Copper(II)oxalate Copper(II) phosphate Iron(II) carbonate Iron(11) fluoride Iron(II) hydroxide Iron(III) hydroxide Iron(III) phosphate Lanthanum hydroxide Lanthanum lodate Lead(11) bromide Lead(11) carbonate Lead(11) chloride Lead(II) fluoride Lead(II) hydroxide Formula AIPO BaCO3 BaCrO BaF Ba(10) Baz(POA)2 BaSO BIASO Cd(AsO4)2 CdCO Cd(CN) CdF Cd(OH) Cd(103) Cd3(PO4)2 CaCO, CaF Ca(OH) Ca(10) C23(PO4)2 CaSO Ce(103)1 Co(ASO) CO3(PO4)2 CuBr CuCl Cul CuSCN Cu3(ASO4)2 Cu(OH) Cu(103)2 CuC0 Cu3(PO4)2 FeCO FeF Fe(OH)2 Fe(OH)3 FePO4 La(OH) La(IO3)3 PbBr PbCO PbCl PbF Pb(OH) Solubility Product 9.8 x 10 21 2.6 x 10-9 1.2 x 10-10 1.8 x 10-7 4.0 x 109 6.0 x 10-19 1.1 x 10-10 4.4 x 100 2.2 x 10-11 1.0 x 10-12 1.0 x 10-8 6.4 x 10- 7.2 x 10-15 2.5 x 10-8 2.5 x 10-3 3.4 x 10 3.5 x 10 11 5.0 x 10 6.5 x 10 2.1 x 10 11 4.9 x 10-5 3.2 x 10-20 6.8 x 10-29 10-1 2.1 x 6.3 10- 1.7 x 107 1.3 x 10-12 1.8 x 10-13 7.9 x 10-6 1.6 x 10 19 7.4 x 10-8 4.4 x 10-0 1.4 x 10-7 3.1 x 10-11 2.4 x 10 4.9 x 10-17 2.8 x 10-9 9.9 x 10- 1.0 x 10-19 75 x 10-2 6.6 x 10 7.4 x 10 4 1.7 x 10-5 3.3 x 10 B 1.4 x 10-20
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
To determine whether a precipitate will form when 200 mL of 10 106 M magnesium chloride is added to ...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Use the solubility product constant from Appendix F to determine whether a precipitate will form if 10 mL 0.0010 M AgNO 3 is added to 10 mL 0.0010 M Na 2 SO 4 . Appendix F Solubility Product...
-
Use the solubility product constant from Appendix F to determine whether a precipitate will form if 25.0 mL of 0.010 M NaOH is added to 75.0 mL of a 0.10 M solution of magnesium chloride? Appendix F...
-
Use the solubility product constant from Appendix F to determine whether a precipitate will form if 10.0 mL of 1.0 10 -6 M iron(II) chloride is added to 20.0 mL of 3.0 10 -4 M barium hydroxide....
-
What is control resolution in a robot positioning system?
-
Companies that make no variable-cost/fixed-cost distinctions must use absorption costing, and those that do make variable-cost/fixed-cost distinctions must use variable costing. Do you agree? Explain.
-
A joint survey by Parade magazine and Yahoo found that 59% of American workers say that if they could do it all over again, they would choose a different career (USA today, September 24, 2012). The...
-
You are faced with making a decision on a large capital investment proposal. The capital investment amount is $\$ 640,000$. Estimated annual revenue at the end of each year in the eight-year study...
-
Logan B. Taylor is a widower whose wife, Sara, died on June 6, 2017. He lives at 4680 Dogwood Lane, Springfield, MO 65801. He is employed as a paralegal by a local law firm. For 2019, he reported die...
-
The following information is from ABC Company's general ledger: Beginning and ending inventories, respectively, for raw materials were $12,000 and $15,000 and for work in process were $30,000 and...
-
Calculate the solubility of copper(II) iodate, Cu(IO 3 ) 2 (K sp = 7.4 10 -8 ), in (a) Water. (b) A 0.10 M copper(II) nitrate solution.
-
Calculate the solubility of barium sulfate (K sp 1.1 10 -10 ) in (a) Water. (b) A 0.10 M barium chloride solution.
-
Every Friday, Max Steadman, Jim Cobun, Lynne Sims, and Tom Hamilton meet at Charley's Food Place after work for refreshments. The four friends work as managers at Eckel Industries, a manufacturer of...
-
Irene and Fred Buckley are age 63 and 66respectively. They have various pensionentitlements and assets. They figure they needabout $45,000 per year after-tax for their lifestyleexpenditures.They want...
-
In 20X2, Carey Hanlon provided tax advice in a letter to Beth Jackson, a tax-only client. For reasons other than the advice given, Jackson engages Riley Urban for tax work in 20X3. Hanlon had not...
-
Accepting only cashless payment forms, such as credit cards or mobile app payments, is an internal control that mitigates the risk of cash being stolen by a cashier in charge of a cash register....
-
Albert purchased Bonzo's car for $500 cash and a non-interest-bearing promissory note for $2,000 payable in two years. How much money can Bonzo expect to receive for the note if he immediately sells...
-
When payment is delayed on a foreign transaction a seller may receive less revenue than expected at time of payment due to the risk of
-
Using the IBM option prices in Figure, calculate the market price of a riskless zero-coupon bond with face value $125 that matures in January on the same date as the listedoptions. PRICES AT CLOSE...
-
Determine by direct integration the values of x for the two volumes obtained by passing a vertical cutting plane through the given shape of Fig. 5.21. The cutting plane is parallel to the base of the...
-
An exhaust system for a room creates a partial vacuum in the room of 1.20 in of water relative to the atmospheric pressure outside the room. Compute the net force exerted on a 36- by 80-in door to...
-
A piece of 14-in Schedule 40 pipe is used as a pressure vessel by capping its ends. Compute the force on the caps if the pressure in the pipe is raised to 325 psig. See Appendix F for the dimensions...
-
A pressure relief valve is designed so that the gas pressure in the tank acts on a piston with a diameter of 30 mm. How much spring force must be applied to the outside of the piston to hold the...
-
Find the current in the 12-2 resistor in the figure below. (Assume R = R3 = 2.2, R ww R www RA R www W 4.0 W 2.00 1202 w www AV
-
1. Iontophoresis. (30 pts) The simplest model for iontophoresis (electrotransport) across the skin involves the so called Nernst-Planck equation, with force due to an electric field on the charged...
-
two hollows spheres of radii 5 cm and 15 cm has an initial charge of 1uC and 10uC, respectively. if the two spheres touch each other , find the charge remaining on the 5 cm sphere?

Study smarter with the SolutionInn App


