Use the standard enthalpies of formation from Appendix G to calculate the enthalpy change for each of
Question:
Use the standard enthalpies of formation from Appendix G to calculate the enthalpy change for each of the following reactions at 298.15 K and 1 atm. Label each as endothermic or exothermic.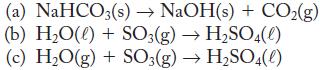
Transcribed Image Text:
(a) NaHCO3(s) → NaOH(s) + CO₂(g) (b) H₂O(l) + SO3(g) →H₂SO4(0) (c) H₂O(g) + SO3(g) → H₂SO4(l)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a 13169 kJ en...View the full answer

Answered By

Robert Mwendwa Nzinga
I am a professional accountant with diverse skills in different fields. I am a great academic writer and article writer. I also possess skills in website development and app development. I have over the years amassed skills in project writing, business planning, human resource administration and tutoring in all business related courses.
4.90+
187+ Reviews
378+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Use the standard enthalpies of formation from Appendix G to calculate the enthalpy change for each of the following reactions at 298.15 K and 1 atm. Label each as endothermic or exothermic. (a) The...
-
The standard enthalpies of formation of ClO and ClO2 are 101 and 102 kJ/mol, respectively. Using these data and the thermodynamic data in Appendix C, calculate the overall enthalpy change for each...
-
The standard free energies of formation and the standard enthalpies of formation at 298 K for difluoroacetylene (C2F2) and hexafluorobenzene (C6F6) are For the following reaction: C6F6(g) 3C2F2(g) a....
-
Vito Co.'s next dividend is expected to be $4.50. Dividend growth is estimated at 20%, 15%, 8% for the following three years, and then stabilize to 2%. How much are you willing to pay to buy one...
-
5,000 lb/h of a saturated aqueous solution of (NH4)2SO4 at 80C is cooled to 30C. At equilibrium, what is the amount of crystals formed in lb/h. If during the cooling process, 50% of the water is...
-
Graph the curve. y = ln (x 2 - 4x + 3)
-
Akur and Aifiti share profits on a 45:55 basis respectively. On 1 July 2025 the equity accounts were as follows. The partners were entitled to 8% interest on capital. Aifiti ran the business and...
-
Return to the example of problem 2. Starting from free trade, assume that Foreign offers exporters a subsidy of 0.5 per unit. Calculate the effects on the price in each country and on welfare, both...
-
18. The atomic number of elements P, Q, R and T are 19,17, 14 and 6 respectively. The pair of elements that can react to form an ionic compound is A. Q and T B. R and Q C. Q and P D. R and T 19. The...
-
Following are the income statement and balance sheet for Medtronic PLC. Consolidated Statement of Income, 12 Months Ended ($ millions) April 26, 2019 Net Sales $30,557 Costs and expenses Cost of...
-
Use data in Appendix G to find the enthalpy of reaction for (a) CaCO3(s) CaO(s) + CO(g) (b) 2HI(g) + F2(g) 2HF(g) + I(s) (c) SF6(g) + 3HO() 6HF(g) + SO3(g)
-
Use standard enthalpies of formation to calculate the enthalpy change for each of the following reactions at 298.15 K and 1 atm. Label each as endothermic or exothermic. (a) The fermentation of...
-
1. Assume you are a financial analyst who works for a major brokerage company that is heavily invested in Bre-X Minerals. a. In what ways would investigating management and directors help determine...
-
Show that if a liquid is in equilibrium with its own vapour and an inert gas in a closed vessel, then \[\frac{\mathrm{d} p_{v}}{\mathrm{~d} p}=\frac{ho_{v}}{ho_{l}}\] where \(p_{v}\) is the partial...
-
A steam turbine operates on a Carnot cycle, with a maximum pressure of 20 bar and a condenser pressure of 0.5 bar. Calculate the salient points of the cycle, the energy addition and work output per...
-
Recalculate P3.1 assuming that the pump efficiency, \(\eta_{\mathrm{P}}=0.8\), and the turbine efficiency, \(\eta_{\mathrm{T}}=\) 0.9. Comment on the effect on the thermal efficiency of the plant,...
-
Show, for a gas obeying the state equation \[p v=(1+\alpha) \Re T\] where \(\alpha\) is a function of temperature alone, that the specific heat at constant pressure is given by \[c_{p}=-\Re T...
-
A cycle is proposed as a development of the Lenoir cycle, in which the working fluid is expanded isentropically from its peak pressure down to a point where its temperature is equal to \(T_{1}\), the...
-
Rework Table under the assumption that the dividend on Fledgling Electronics is $10 next year and that it is expected to grow by 5% a year. The capitalization rate is15%. Expected Future Values...
-
Information graphics, also called infographics, are wildly popular, especially in online environments. Why do you think infographics continue to receive so much attention? How could infographics be...
-
Describe the changes in a beaker containing water and butanol that you would observe along the path f j k in Figure 19.24b. How would you calculate the relative amounts of different phases present...
-
Describe the system at points a and c in Figure 19.25b. How would you calculate the relative amounts of different phases present at these points?
-
What information can be obtained from a tie line in a PZ phase diagram?
-
Imagine that you are planning to make a big purchase. You could be buying a car, a house, a boat, or any other item that costs many thousand dollars (feel free to be creative). Suppose you need to...
-
Zulu sells its waterproof phone case for $125 per unit. Fixed costs total $242,000, and variable costs are $50 per unit. Compute the units that must be sold to get a target income of $214,000....
-
Sandstorm Entertainment needs $684,000 to take a cash discount of 2.50/10, net 80. A banker will loan the money for 70 days at an interest cost of $15,400. a. What is the annual rate on the bank...

Study smarter with the SolutionInn App


