Use standard enthalpies of formation to calculate the enthalpy change for each of the following reactions at
Question:
Use standard enthalpies of formation to calculate the enthalpy change for each of the following reactions at 298.15 K and 1 atm. Label each as endothermic or exothermic.
(a) The fermentation of glucose to ethyl alcohol and carbon dioxide:![]()
(b) The combustion of normal (straight-chain) butane: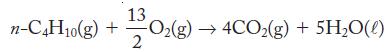
Transcribed Image Text:
C6H1206(s) → 2C₂H5OH() + 2CO₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a 74 kJ ...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
An important step in the production of sulfuric acid is the oxidation of SO 2 to SO 3 . Formation of SO 3 from the air pollutant SO 2 is also a key step in the formation of acid rain. (a) Use...
-
Use standard enthalpies of formation to calculate the enthalpy change that occurs when 1.00 g of SnCl 4 () reacts with excess H 2 O() to form SnO 2 (s) and HCl(aq).
-
Use the standard enthalpies of formation from Appendix G to calculate the enthalpy change for each of the following reactions at 298.15 K and 1 atm. Label each as endothermic or exothermic. (a)...
-
The owner of a smoky bar in a warm climate relies on natural exchange between the bar and outside to keep smoke levels manageable in the bar (below 50 microgram/m). Smokers account for a smoke PM10...
-
A screen analysis for a sample of glauber's salt from a commercial crystallizer is as follows, where the crystals can be assumed to have a uniform sphericity and volume shape factor. Use a...
-
Find the most general antiderivative or indefinite integral. You may need to try a solution and then adjust your guess. Check your answers by differentiation. (-2 cos t) dt
-
Meagan and Jenny are in partnership, sharing profits equally. Provision exists in the partnership agreement for charging interest on capital at the rate of 8% p.a. and interest on drawings at 10%...
-
Elite Apparel Inc. is considering two investment projects. The estimated net cash flows from each project are as follows: Each project requires an investment of $900,000. A rate of 15% has been...
-
26 The number of protons, neutrons, electrons in some particles are shown in the table below Particle Protons Neutrons electrons P 1 1 2 Q 2 2 2 R 3 4 2 T 4 5 4 Which one of the following particles...
-
An army division in Iraq has five troop encampments in the desert, and the division leaders want to determine the best location for a supply depot to serve the camps. The (x, y) coordinates (in...
-
Use the standard enthalpies of formation from Appendix G to calculate the enthalpy change for each of the following reactions at 298.15 K and 1 atm. Label each as endothermic or exothermic. (a) The...
-
Write the chemical equation for the reaction whose energy change is the standard enthalpy of formation of each of the following substances. (a) CHCOOH(e) (b) HPO4(0) (c) CaSO4 2HO(s) (d) C(s, diamond)
-
How does a virtual machine (VM) function?
-
The Dieterici equation for a pure substance is given by \[p=\frac{\Re T}{v-b} \mathrm{e}^{-\frac{a}{\Re T v}}\] Determine (a) the constants \(a\) and \(b\) in terms of the critical pressure and...
-
Derive expressions for \(\left(\frac{\partial c_{v}}{\partial v} ight)_{T}\) for substances obeying the following laws: (a) \(p=\frac{\Re T}{v-b} \mathrm{e}^{-\frac{a}{\Re T v}}\) (b) \(p=\frac{\Re...
-
Recalculate P3.2 assuming that the pump efficiency \(\eta_{\mathrm{P}}=0.8\), and the turbine efficiency \(\eta_{\mathrm{T}}=\) 0.9. Comment on the effect on the thermal efficiency of the plant, and...
-
A piston-cylinder assembly contains \(3 \mathrm{~kg}\) of air at 15 bar and \(620 \mathrm{~K}\). The environment is at a pressure of 1 bar and \(300 \mathrm{~K}\). The air is expanded in a fully...
-
Show \[\begin{aligned} \text { (a) } T \mathrm{~d} s & =c_{p} \mathrm{~d} T-T\left(\frac{\partial v}{\partial T} ight)_{p} \mathrm{~d} p \\ \text { (b) } T \mathrm{~d} s & =c_{v}\left(\frac{\partial...
-
What do financial managers mean by free cash flow? How is free cash flow calculated? Briefly explain.
-
Provide a few individual examples who revealed what aspects of emotional intelligence?
-
Calculate I, , and a for a 0.0175 m solution of Na 3 PO 4 at 298 K. Assume complete dissociation. How confident are you that your calculated results will agree with experimental results?
-
Calculate the ionic strength of each of the solutions in Problem P10.4. In Problem V-l, + V-H- Usolute
-
Calculate the value of m in 5.5 10 3 molal solutions of (a) KCl (b) Ca(NO 3 ) 2 (c) ZnSO 4 . Assume complete dissociation.
-
1. Which segmentation variable(s) would you use if you were to segment the global market for Louis Vuitton? 2. China has been a major market for many luxury brands such as Louis Vuitton (LV),...
-
Develop a financial plan for either an actual small business or a hypothetical one. This should be the same company you introduced in your Week 3 Executive Summary Assignment response. For this...
-
24. Li hired Sally, a real estate agent, to sell Li's block of land. Li wanted at least $100,000 for the land. A few weeks later, Sally tod Li that her husband would buy the land for $100,000. After...

Study smarter with the SolutionInn App


