What is the equilibrium constant for the following reaction? Strategy Determine the number of electrons transferred, then
Question:
What is the equilibrium constant for the following reaction?![]()
Strategy Determine the number of electrons transferred, then use Equation 18.4.
Equation 18.4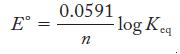
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
The standard voltage of the reaction i...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
4. Define a class Person according to the following UML: Person -name: String -age: int + Person (name: String, age: int) + setName (name: String): void + getName(): String + setAge (age: int) : void...
-
What is the equilibrium constant for the following reaction at 25°C? Mg(s) + Zn2+ (aq)--Mg2+ (aq) + Zn(s)
-
At what annual interest rate, compounded annually, would $500 have to be invested for it to grow to $1,892.84 in 12 years? Question content area bottom Part 1 The annual interest rate, compounded...
-
Suppose that you borrow $1000.00 from a friend and promise to pay back $1975.00 in 5 years. What simple interest rate will you pay?
-
The spectral absorptivity ?? and spectral reflectivity p? for a spectrally selective, diffuse material are as shown. (a) Sketch the spectral transmissivity ??. (b) If solar irradiation with GS = 750...
-
A diagram for an open- tube manometer is shown below. If the flask is open to the atmosphere, the mercury levels are equal. For each of the following situations in which a gas is contained in the...
-
Describe the different types of events and compare them.
-
Orion Corporation has established the following standards for the prime costs of one unit of its chief product, dartboards. Standard Quantity Standard Price or Rate Standard Cost Direct...
-
Find the sources of finance for Netflix, Amazon prime, alt Balaji, Zee 5 and Disney hotstar .find It's capital structure, its operating leverage, financial leverage, combined leverage and company...
-
A voltaic cell consists of a half-cell of iron ions in a solution with [Fe2 + ] = 2.0 M and [Fe3 + ] = 0.75 M , and a half-cell of copper metal immersed in a solution containing Cu 2+ at a...
-
Calculate the standard free energy change for the reaction Strategy Use Table 18.1 to determine the cell potential; then use Equation 18.2 to calculate the G. You will also have to determine the...
-
Select the correct answer for each of the following questions. 1. A major impact of the Foreign Corrupt Practices Act of 1977 is that registrants subject to the Securities Exchange Act of 1934 are...
-
Compute the effect of the new lease accounting standard on the following measures for American Airlines in 2019 (in other words, how different would these measures be under the old accounting...
-
think about an organizational improvement initiative that you would like to make in an organization of your choice. This initiative might focus on improved profitability, better customer service, new...
-
The velocity of an airplane flying into a headwind is given by v(t) = 30 (25-t2) mi/hr for 0 st4 hr. Assume that s(0) = 0. a. Determine the position function for Osts4. b. How far does the airplane...
-
The definition of a liability forms an important element of the International Accounting Standards Board's Framework for the Preparation and Presentation of Financial Statements which, in turn, forms...
-
The senior on a CPA firm's largest audit engagement received a request from the client's CFO for a copy of "any communications the firm has sent relating tointernal-control-relatedmatters identified...
-
Explain how an auditor should link control deficiencies to the design of audit tests. Identify the logic process utilized by the auditor to make the linkage.
-
An educational researcher devised a wooden toy assembly project to test learning in 6-year-olds. The time in seconds to assemble the project was noted, and the toy was disassembled out of the childs...
-
A simple soft drink system relies on pressurized CO 2 to force the soft drink (sg = 1.08) from its tank sitting on the floor up to the outlet where cups are filled. Determine the required CO 2...
-
A spa tub is to be designed to replace bath tubs in renovations. There are to be 6 outlet nozzles, each with a diameter of 12 mm, and each should have an outlet velocity of 12 m/s. What is the...
-
A village currently carries water by hand from a lake that is 1200 m from the village center. It is later determined that the surface of the lake is 3 m above the elevation of the village, so someone...
-
Preparing a chart of accounts and opening an account Lillian Deters owns a service business called Deters Duplicating, which uses the following accounts. Instructions: 1. Prepare a chart of accounts...
-
How do individual differences in decision-making styles, such as risk propensity, tolerance for ambiguity, and time orientation, impact the quality and outcomes of decisions, and how can these...
-
Mixers Ltd. is engaged in producing a standard mix using 60 kg of chemical X and 40 kg of chemical Y. The standard loss of production is 30%. The standard price of X is Rs.5 per kg and of Y is Rs.10...

Study smarter with the SolutionInn App


