Question: What quantity of heat must be added to a 120-g sample of aluminum to change its temperature from 23.0 C to 34.0 C ? Strategy
What quantity of heat must be added to a 120-g sample of aluminum to change its temperature from 23.0 °C to 34.0 °C ?
Strategy
Use Equation 5.4 and the value of the specific heat of aluminum from Table 5.1 to determine the heat.
Equation 5.4![]()
Table 5.1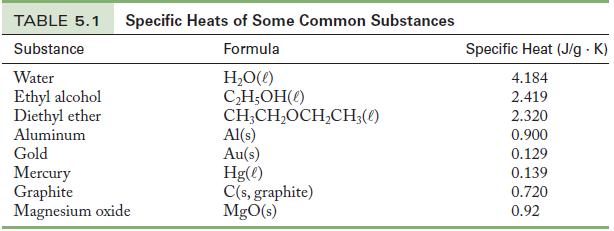
g=mC AT mC.AT
Step by Step Solution
3.43 Rating (153 Votes )
There are 3 Steps involved in it
First we need the temperature ... View full answer

Get step-by-step solutions from verified subject matter experts


