What quantity of heat must be added to a 120-g sample of aluminum to change its temperature
Question:
What quantity of heat must be added to a 120-g sample of aluminum to change its temperature from 23.0 °C to 34.0 °C ?
Strategy
Use Equation 5.4 and the value of the specific heat of aluminum from Table 5.1 to determine the heat.
Equation 5.4![]()
Table 5.1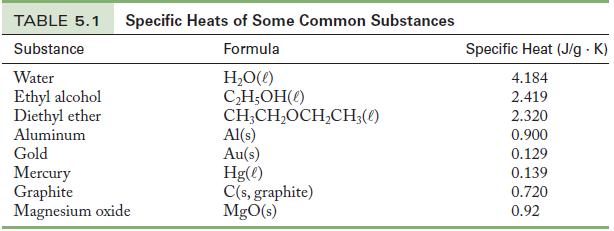
Transcribed Image Text:
g=mC AT mC.AT
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
First we need the temperature ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
1. How many Btu of heat must be added to 1200 lb of copper to raise its temperature from 100F to 450F? 2. How many Btu of heat are given off by 500 lb of aluminum when it cools from 650F to 75F? 3....
-
How many kcal of heat must be added to 148 kg of aluminum to raise its temperature from 21.5C to 485C?
-
Heat is added isothermally to 2.5 mol of a monatomic ideal gas. The temperature of the gas is 430 K. How much heat must be added to make the volume of the gas double?
-
The two roots of a quadratic equation ax 2 + bx + c = 0 can be obtained using the following formula: b 2 - 4ac is called the discriminant of the quadratic equation. If it is positive, the equation...
-
The separation of air into nitrogen and oxygen is widely practiced. Cryogenic distillation is most economical for processing 100 to 5,000 tons of air per day, while pressure-swing-adsorption is...
-
Determine whether the statement is true or false. If it is false, explain why or give an example that shows it is false. ln(a n+m ) = n ln a + m ln a, where a > 0 and m and n are rational.
-
Krista was a passenger in a rented van. She sustained injuries when the driver of the van slammed into a tree after the vans brakes failed. The driver of the van was not an authorized driver pursuant...
-
A traveling production of Fiddler on the Roof performs each year. The average show sells 1,200 tickets at $ 55 a ticket. There are 115 shows each year. The show has a cast of 60, each earning an...
-
You can find this question from Excel worksheet "poison pill question". Twilight has 1,000,000 shares outstanding, and the current stock price is $25 per share. Yesterday, Activist Apollo made a...
-
A venturi meter similar to the one in Fig. 15.2 is attached to a 4-in Schedule 40 steel pipe and has a throat diameter of 1.50 in. Determine the pressure difference across the meter that would be...
-
What is the difference between the enthalpy of reaction and the enthalpy of formation? For what chemical reaction(s) are the two quantities the same?
-
Why is chemical energy classified as a form of potential energy?
-
Catching the Bus a student is running at her top speed of 5.0 m/s to catch a bus, which is stopped at the bus stop. When the student is still 40.0 m from the bus, it starts to pull away, moving with...
-
Find the state vector via the formal-solution approach. \(\dot{\mathbf{x}}=\left[\begin{array}{cc}1 & -1 \\ -4 & 1\end{array} ight] \mathbf{x}+\left[\begin{array}{c}1 \\ \frac{1}{2}\end{array} ight]...
-
\(\mathbb{A}\) Consider a second-order system \(Y(s) / U(s)=\omega_{n}^{2} /\left(s^{2}+2 \zeta \omega_{n} s+\omega_{n}^{2} ight)\). a. Write a MATLAB m-file to plot the magnitude of the system's...
-
A single-tank liquid-level system with inflow rate \(q_{i}\) as its input and liquid level \(h\) as its output is modeled as \(R A \dot{h}+g h=R q_{i}(t), h(0)=0\), where \(R, A, g=\) const. If the...
-
The mathematical model of a dynamic system is described by \[4 \ddot{x}+4 \dot{x}+5 x=\frac{10}{3} u_{r}(t), \quad x(0)=0, \quad \dot{x}(0)=\frac{2}{3}\] where \(u_{r}(t)\) is the unit ramp. Plot the...
-
Find the state vector via the formal-solution approach. \(\dot{\mathbf{x}}=\left[\begin{array}{cc}5 & 1 \\ -4 & 1\end{array} ight] \mathbf{x}+\left[\begin{array}{c}1 \\ -1\end{array} ight] u, \quad...
-
The stock price of Heavy Metal (HM) changes only once a month: either it goes up by 20% or it falls by 16.7%. Its price now is $40. The interest rate is 12.7% per year, or about 1% per month. a. What...
-
Use of the contraceptive Depo Provera appears to triple women's risk of infection with chlamydia and gonorrhea , a study reports today. An estimated 20 million to 30 million women worldwide use Depo...
-
For a pair of keto-enol tautomers, explain how IR spectroscopy might be used to identify whether the equilibrium favors the ketone or the enol.
-
Acrolein is an α,β-unsaturated aldehyde that is used in the production of a variety of polymers. Acrolein can be prepared by treating glycerol with an acid catalyst. Propose...
-
Draw the structure of the product with molecular formula C 10 H 10 O that is obtained when the compound below is heated with aqueous acid. CN CN C10H100 Heat
-
How do endocrine glands integrate diverse physiological signals and regulate systemic homeostasis through the secretion of hormones, coordinating intricate intercellular communication and adaptive...
-
How do circadian rhythms, governed by the central circadian clock and peripheral oscillators within endocrine tissues, regulate the temporal patterns of hormone secretion, tissue responsiveness, and...
-
Dell Technologies, Inc. FREE CASH FLOW STATEMENT 2022, 2021, 2020 All Data is in Millions Free Cash Flow Calculation: Cash Flow from Operations: Less: Capital Expenditures: Free Cash Flow: $ 2022...

Study smarter with the SolutionInn App


