Figure 7.4 shows the radial probability distribution functions for the 2s orbitals and 2p orbitals. (a) Which
Question:
Figure 7.4 shows the radial probability distribution functions for the 2s orbitals and 2p orbitals.
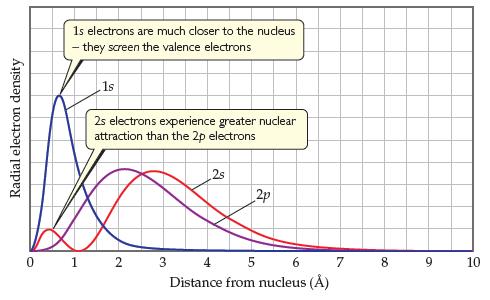
(a) Which orbital, 2s or 2p, has more electron density close to the nucleus?
(b) How would you modify Slater’s rules to adjust for the difference in electronic penetration of the nucleus for the 2s and 2p orbitals?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry The Central Science
ISBN: 9780321910417
13th Edition
Authors: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus
Question Posted:





