Convert an absolute pressure of 7.00 10 5 N/m 2 to gauge pressure in lb/in 2
Question:
Convert an absolute pressure of 7.00 × 105 N/m2 to gauge pressure in lb/in2. (This value was stated to be just less than 90.0 lb/in2 in Example 13.9. Is it?)
Data given in Example 13.9
How many moles of gas are in a bike tire with a volume of 2.00 x 10-3 m3 (2.00 L), a pressure of 7.00 x 105 Pa (a gauge pressure of just under 90.0 lb/in2), and at a temperature of 18.0°C?
Strategy
Identify the knowns and unknowns, and choose an equation to solve for the unknown. In this case, we solve the ideal gas law, PV = nRT, for the number of moles n.
Data given in Example 13.6
Suppose your bicycle tire is fully inflated, with an absolute pressure of 7.00 x 105 Pa (a gauge pressure of just under 90.0 lb/in2) at a temperature of 18.0°C. What is the pressure after its temperature has risen to 35.0°C? Assume that there are no appreciable leaks or changes in volume.
Strategy
The pressure in the tire is changing only because of changes in temperature. First we need to identify what we know and what we want to know, and then identify an equation to solve for the unknown.
We know the initial pressure P0=7.00 x 105 Pa, the initial temperature T0 = 18.0C, and the final temperature Tf = 35.0°C. We must find the final pressure Pf. How can we use the equation PV = NkT? At first, it may seem that not enough information is given, because the volume V and number of atoms N are not specified. What we can do is use the equation twice: P0V0 = NkT0 and PfVf = NkTf. If we divide PfVf by P0V0 we can come up with an equation that allows us to solve for Pf.
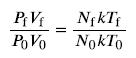
Since the volume is constant, Vf and V0 are the same and they cancel out. The same is true for Nf and N0, and k, which is a constant. Therefore,
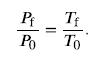
We can then rearrange this to solve for Pf:
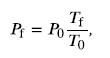
where the temperature must be in units of kelvins, because T0 and Tf are absolute temperatures.
Step by Step Answer:






