What pressure would be created in the gasoline tank considered in Example 13.4, if the gasoline increases
Question:
What pressure would be created in the gasoline tank considered in Example 13.4, if the gasoline increases in temperature from 15.0°C to 35.0°C without being allowed to expand? Assume that the bulk modulus B for gasoline is 1.00 x 109 N/m2. (For more on bulk modulus, see Elasticity: Stress and Strain.)
Strategy
To solve this problem, we must use the following equation, which relates a change in volume ΔV to pressure:
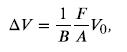
where F/A is pressure, V0 is the original volume, and B is the bulk modulus of the material involved. We will use the amount spilled in Example 13.4 as the change in volume, ΔV.
Data given in Example 13.4
Suppose your 60.0-L (15.9-gal) steel gasoline tank is full of gas, so both the tank and the gasoline have a temperature of 15.0°C. How much gasoline has spilled by the time they warm to 35.0°C?
Strategy
The tank and gasoline increase in volume, but the gasoline increases more, so the amount spilled is the difference in their volume changes. (The gasoline tank can be treated as solid steel.) We can use the equation for volume expansion to calculate the change in volume of the gasoline and of the tank.
Step by Step Answer:






