How readily an acid donates a hydrogen ion is a function of how well the acid is
Question:
How readily an acid donates a hydrogen ion is a function of how well the acid is able to accommodate the resulting negative charge it gains after donating. Which should be the stronger acid: water or hypochlorous acid? Explain.
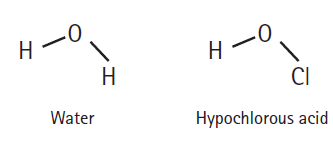
Transcribed Image Text:
0- H- 0- CI Н Hypochlorous acid Water
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 88% (9 reviews)
These molecules behave as acids by losing the hydrogen ion from the oxygen atom which takes o...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Conceptual Physical Science
ISBN: 978-0134060491
6th edition
Authors: Paul G. Hewitt, John A. Suchocki, Leslie A. Hewitt
Question Posted:
Students also viewed these Physics questions
-
A Friedel-Crafts acylation is an electrophilic aromatic substitution in which the electrophile (E + ) is an acylium ion. There are other methods of forming acylium ions, such as treatment of an...
-
In the following acid-base reactions, 1. Determine which species are acting as electrophiles (acids) and which are acting as nucleophiles (bases). 2. Use the curved-arrow formalism to show the...
-
Chemists know that nitric and sulfuric acids are strong acids and that acetic acid is a weak acid. They would also agree that ethanol is at best a very weak acid. Acid strength is given directly by...
-
Molly earns a gross yearly salary of $132,676. She has no dependent children and made the following tax deductible purchases: Charitable contributions: $5,401 Student loan interest: $1,429 When she...
-
Jerry, who is single with no dependents and does not itemize, provides you with the following information for the tax year. Short-term capital loss...
-
The Milken Investment Fund buys 90 bonds of the Levine Corporation through its broker. The bonds pay 11 percent annual interest. The yield to maturity (market rate of interest) is 14 percent. The...
-
Paul Taylor has recently purchased a brand new, top-of-the-range Mercedes car. He works as a junior cashier receiving cash, cheque and credit payments for a retail furniture store. Recently a large...
-
SallyMay, Inc., designs and manufactures T-shirts. It sells its T-shirts to brand-name clothes retailers in lots of one dozen. SallyMays May 2013 static budget and actual results for direct inputs...
-
Need help with SQL homework, questions start under the problems section. This document is intended to relate the concepts we discuss in class to the Oracle SQL Language Reference or to the Oracle...
-
An ISP is granted the block 80.70.56.0/21. The ISP needs to allocate addresses for two organizations each with 500 addresses, two organizations each with 250 addresses, and three organizations each...
-
Which should be the stronger base: ammonia, NH 3 , or nitrogen trifluoride, NF 3 ? F- N-F H - N-H Ammonia Nitrogen trifluoride
-
The main component of bleach is sodium hypochlorite, NaOCl, which consists of sodium ions, Na + , and hypochlorite ions, - OCl. What products are formed when this compound reacts with the...
-
How does the temperature equation simplify if there is no flow? Write this out in detail in all of the three coordinate systems.
-
What are some things, or strategies, a person can do to develop a good speaking voice and make eye contact?
-
How can you use the internet effectively in your job search?
-
What are three ways to create a professional image with your letter?
-
What are two types of rsums, and when should you use each?
-
A student has written the following recommendation section for a report for a local restaurant. The restaurant is called the American Grill and specializes in burgers and fries. The restaurant is new...
-
(a) If two sounds differ by 5.00 dB, find the ratio of the intensity of the louder sound to that of the softer one. (b) If one sound is 100 times as intense as another, by how much do they differ in...
-
Southwestern Punch was made by Frutayuda, Inc. and sold in 12-ounce cans to benefit victims of Hurricane Zero. The mean number of ounces placed in a can by an automatic fill pump is 11.7 with a...
-
You have been hired to consult on a pilot plant project that requires the installation of a rough horizontal pipe made from commercial steel into a flow system. The pipe has a diameter of 4 in. and a...
-
Your boss comes to you with an important project where you are to determine the pressure drop in a rough horizontal high-pressure pipe made of cast iron. The pipe has a diameter of 0.25 ft and a...
-
Freon-12 is flowing at 10 ft/sec through a pipe made of galvanized iron at 100F. If the pipe is 100 ft long and 4 in. in diameter, what is the head loss in this system?
-
1. Write a Java program that will prompt the user for a number and print out a square with those dimensions. For example, if the user enters 5, return the following: * * * ** ** * * * * * * * * * * *...
-
2. Vector multiplication or dot product is performed by multiplying corresponding elements and summing the products. It can be represented mathematically as oa; *bi where a and b are vectors of...
-
The following data was collected from an experiment testing the hypothesis: The density of cream whipped for 5 minutes increases at higher altitudes. What would be an acceptable interpretation of...

Study smarter with the SolutionInn App


