In a simple reaction A A*, a molecule is interconvertible between two forms that differ in
Question:
In a simple reaction A ↔ A*, a molecule is interconvertible between two forms that differ in standard free energy G° by 18 kJ/mole, with A* having the higher G°.
A. Use Table 3–1 to find how many more molecules will be in state A* compared with state A at equilibrium.
B. If an enzyme lowered the activation energy of the reaction by 11.7 kJ/mole, how would the ratio of A to A* change?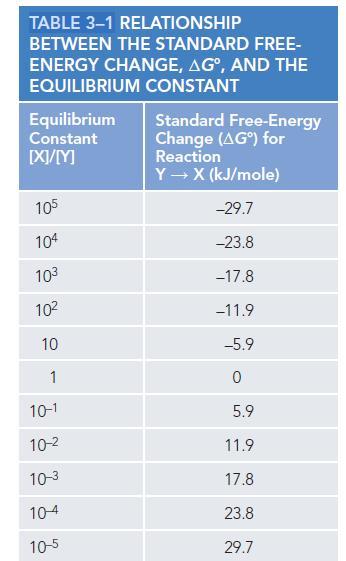
Transcribed Image Text:
TABLE 3-1 RELATIONSHIP BETWEEN THE STANDARD FREE- ENERGY CHANGE, AG°, AND THE EQUILIBRIUM CONSTANT Equilibrium Constant [X]/[M] 105 104 103 10² 10 1 10-1 10-² 10-3 10-4 10-5 Standard Free-Energy Change (AG) for Reaction Y→ X (kJ/mole) -29.7 -23.8 -17.8 -11.9 -5.9 0 5.9 11.9 17.8 23.8 29.7
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
A To find how many more molecules will be in state A compared to state A at equilibrium we need to u...View the full answer

Answered By

Surendar Kumaradevan
I have worked with both teachers and students to offer specialized help with everything from grammar and vocabulary to challenging problem-solving in a range of academic disciplines. For each student's specific needs, I can offer explanations, examples, and practice tasks that will help them better understand complex ideas and develop their skills.
I employ a range of techniques and resources in my engaged, interesting tutoring sessions to keep students motivated and on task. I have the tools necessary to offer students the support and direction they require in order to achieve, whether they need assistance with their homework, test preparation, or simply want to hone their skills in a particular subject area.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Essential Cell Biology
ISBN: 9780393680362
5th Edition
Authors: Bruce Alberts, Karen Hopkin, Alexander Johnson, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Read the case study "Southwest Airlines," found in Part 2 of your textbook. Review the "Guide to Case Analysis" found on pp. CA1 - CA11 of your textbook. (This guide follows the last case in the...
-
Identify a true statement about the rational and emotional aspects of leadership. Multiple choice question. Leadership is not about the rational or emotional sides of human experience Leadership...
-
Data for Virtual Gaming Systems are provided in P124A. Earnings per share for the year ended December 31, 2015, are $1.40. The closing stock price on December 31, 2015, is $28.30. In P124A, The...
-
1. What are the internationalisation drivers Walmart International has struggled with? 2. What might be the dangers for a large Western retailer in staying out of emerging markets?
-
Based on the design, briefly discuss the data collection procedures to be used. Be sure to include the area rea of focus and targeted sample as part of these procedures. Develop a hypothetical...
-
Trevor Corporations balance sheet at December 31, 2013, is presented below. During 2014, the following transactions occurred. 1. Trevor paid $2,500 interest on the bonds on January 1, 2014. 2. Trevor...
-
Supposons un consommateur ayant une richesse W qui est distribue selon une loi de densit de probabilit fw (w). Montrez qu'on peut obtenir une approximation du cot du risque, CR, en utilisant: 1 CRrr...
-
Given the project information in the following table, what is the probability of completing the National Holiday Toy project in 100-time units? Hint: Use the =NORM.S.DIST(z. TRUE) function in Excel...
-
Which of the following amino acids would you expect to find more often near the center of a folded globular protein? Which ones would you expect to find more often exposed to the outside? Explain...
-
Neurofilament proteins assemble into long, intermediate filaments, found in abundance running along the length of nerve cell axons. The C-terminal region of these proteins is an unstructured...
-
All business transactions affect assets (resources owned), liabilities (amounts owed), stockholders equity (ownership interest), or some combination of these items. Following is an analysis of the...
-
How is the total budgeted purchases for a retail business calculated?
-
State an acceptable standard (benchmark) you would like to achieve. For example, if your identified behaviour is an absenteeism rate of 12%, then you might see 7% being a standard.
-
Zain Hakim's client does not have in place appropriate controls for a risk he has identified. What should Zain do?
-
With the accounting principle of prudence how do some businesses run outside of the conservative nature that we see under this accounting principle? How do the businesses take the chances to make...
-
Mario and Peter just opened a cleaning services business. Mario is saying that revenue must be recognized each time a cleaning service has been rendered. On the other hand, Peter is arguing that...
-
The owner of a drugstore is wondering whether he should target female customers in particular, because he believes that they tend to spend more than male customers. He asks the cashiers to keep track...
-
Discuss the information available from the following techniques in the analysis of inorganic pigments used in antique oil paintings: (i) Powder X-ray diffraction, (ii) Infrared and Raman...
-
In each case, identify the more stable anion. Explain why it is more stable. (a) (b) (c) vs. N. vs. -zo
-
Atropine, extracted from the plant Atropa belladonna, has been used in the treatment of bradycardia (low heart rate) and cardiac arrest. Draw the enantiomer of atropine: CH 0= -
-
Recall that a C=C bond is comprised of a s bond and a p bond. These two bonds together have a combined BDE (bond dissociation energy) of 632 kJ/mol. Use this information to predict whether the...
-
The store manager of a local independent grocery store thought customers might stay in the store longer if slow, easy-to-listen to music were played over the stores intercom system. After some...
-
If the force applied to the handle of the load binder is 50 lb, determine the tensions T and T in each end of the chain and then draw the shear and moment diagrams for the arm ABC. 750 lb -12 in. B 3...
-
Draw the shear and moment diagrams for the beam, and determine the shear and moment throughout the beam as functions of x for 0 x 6 ft and 6 ft x 10 ft. 2 kip/ft 10 kip 8 kip 40 kip-ft 6 ft 4 ft

Study smarter with the SolutionInn App


