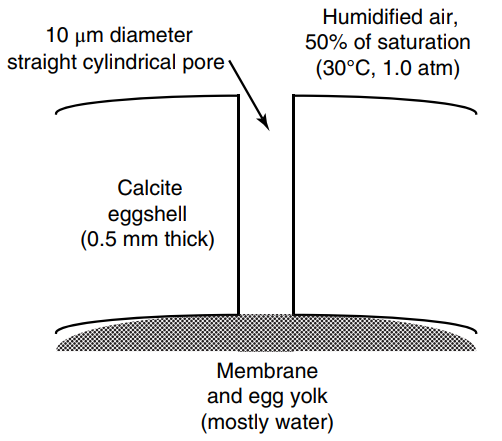
Chicken eggs possess a hard, porous shell of calcite mineral. Cylindrical pores of 10 micron (µm) diameter
Question:
a. What is the molecular diffusion coefficient of water vapor in air at 30oC and 1.0 atm inside the pore? Is Knudsen diffusion very important in this diffusion process?
b. Determine the water loss from a single egg in units of grams of water per day. State all relevant assumptions used in the calculation.
c. Propose two changes in process conditions within the hen house that would reduce water loss by at least 50%.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Momentum Heat And Mass Transfer
ISBN: 9781118947463
6th Edition
Authors: James Welty, Gregory L. Rorrer, David G. Foster
Question Posted:





