A compound has a molecular mass of 120 g/mol, and the information in the table below is
Question:
A compound has a molecular mass of 120 g/mol, and the information in the table below is the only other data available for a compound. Fill in all of the empty cells with your best estimate of the value. Explain any assumptions or approximations you make.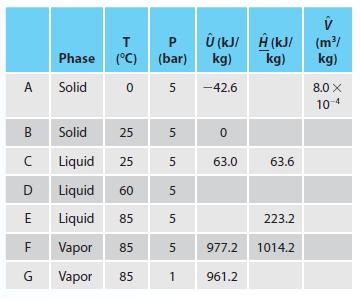
Transcribed Image Text:
A B с D E F G T Phase (°C) (°C) Solid 0 Solid 25 Liquid 25 Liquid 60 Liquid 85 Vapor 85 Vapor 85 P (bar) 5 5 сл 5 5 5 5 1 сл Û (kJ/ kg) -42.6 0 63.0 977.2 961.2 V Ĥ (kJ/(m²/ kg) kg) 63.6 223.2 1014.2 8.0 X 10-4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
Here are my best estimates for the empty cells in the tablebased on the information provided Phase T ...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
Given below are the zeros and its multiplicities, write the function in factored form Zeros: 4 mult 1 -1 mult 2 -5 mult 3
-
THIRD AVENUE SOFTWARE HEALTH-CARE APP PROJECT This case is new for the ninth edition of Information Technology Project Management . The case provides an opportunity to apply agile and Scrum...
-
State whether or not each of the following events would result in a liability being recognised in the accounts at 30 June. 1. Taxes for the year ended 30 June, which are not payable until October. 2....
-
On January 1, 2010, Turner Construction Company agreed to construct an observatory for Dartmouth College for $120 million. Dartmouth College must pay $30 million upon signing and $30 million at the...
-
Perform an experimental analysis of the efficiency (number of character comparisons performed) of the brute-force and Boyer-Moore pattern-matching algorithms for varying-length patterns.
-
If the boundary layer on the hood of your car behaves as one on a flat plate, estimate how far from the front edge of the hood the boundary layer becomes turbulent. How thick is the boundary layer at...
-
Taussig Technologies Corporation (TTC) has been growing at a rate of 20% per year in recent years. This same supernormal growth rate is expected to last for an other 2 years (g1 = g2 20%) (a) If D0 =...
-
Describe the principal advantages of alternative dispute resolution. While a mediated settlement is not binding on the parties, it brings with it certain benefits. What are they? What are the...
-
10 m 3 of saturated steam at T = 150C is mixed with 0.1 m 3 of saturated liquid water at T = 150C. How many total kilograms of H 2 O does the mixture contain?
-
Model water using the van der Waals equation of state with a = 5.53 10 6 bar cm 6 /mol 2 and b = 30.48 cm 3 /mol (The values of the van der Waals a and b were determined using the method...
-
As leaders make strategic decisions, they must balance the interests of various stakeholders employees, customers, shareholders, suppliers, unions/activists, and the community. Describe the best...
-
Consider that the current world price for copper ore it's $3.90 per pound. Suppose that domestic market for copper ore in Chile is described by the following demand and supply equation respectively P...
-
A wise guy majoring in math says that he thinks your lottery is a bad deal.What other utility might you tell the wise guy he might receive that makes the lottery worth playing?
-
Q1. Please make the correct statement: The argument that ETFs might exacerbate the volatility of assets in markets ... Select one: A. Only applies in a bullish market B. applies in any market...
-
What makes a monetary policy "unconventional"? Make a chronological analysis of advanced economies' monetary policies since the global financial crisis of 2007-2009, including the Covid-19 pandemic...
-
Year 3 calculate the additional amount of synergies (beyond the $100 million already included in the projections) needed to get to zero dilution or accretion if the number of share outstanding after...
-
Research by Harvard Medical School experts suggests that boys are more likely than girls to grow out of childhood asthma when they hit their teenage years (BBC News, August 15, 2008). Scientists...
-
Interview managers at three companies in your area about their use of ERP. How have their experiences been similar? What accounts for the similarities and differences?
-
The radius of an input pinion is 3.8 cm, and the radius of an output gear is 11.4 cm. Calculate the velocity and torque ratios of the gearset.
-
The torque ratio of a gearset is 0.75. The pinion gear has 36 teeth and a diametral pitch of 8. Determine the number of teeth on the output gear and the radii of both gears.
-
You are designing a geartrain with three spur gears: one input gear, one idler gear, and one output gear. The diametral pitch for the geartrain is 16. The diameter of the input gear needs to be twice...
-
1. Briefly describe the Balanced Scorecard approach to control. Explain how it differs from the traditional approach to control used in most companies. 2. Briefly differentiate between active...
-
Discuss how research can help the company meet those consumer trends. How do companies use evidence in management decisions (evidence-based management) to support strategic choices?
-
Discuss three social and legal concerns for the internet and e-business. Be specific and provide examples

Study smarter with the SolutionInn App


