Model water using the van der Waals equation of state with a = 5.53 10 6
Question:
Model water using the van der Waals equation of state with a = 5.53 × 106 bar · cm6/mol2 and b = 30.48 cm3/mol (The values of the van der Waals a and b were determined using the method illustrated in Example 7-4).
A. Make a graph of P vs. V ˆ for water at T = 100°C. Use the data from the steam tables.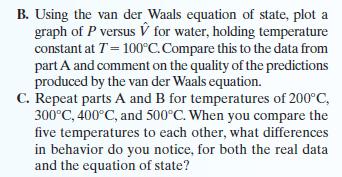
Transcribed Image Text:
B. Using the van der Waals equation of state, plot a graph of P versus V for water, holding temperature constant at T = 100°C. Compare this to the data from part A and comment on the quality of the predictions produced by the van der Waals equation. C. Repeat parts A and B for temperatures of 200°C, 300°C, 400°C, and 500°C. When you compare the five temperatures to each other, what differences in behavior do you notice, for both the real data and the equation of state?
Step by Step Answer:

This question has not been answered yet.
You can Ask your question!
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
The van der Waals equation of state is where a and b are temperature-independent parameters that have different values for each gas. For carbon dioxide, a = 0.3640 Pa m 6 mol 2 and b = 4.267 Ã...
-
The van der Waals equation of state, an approximate representation of the behavior of gases at high pressure, is given by Where a and b are constants having different values for different gases. (In...
-
The overall goal of this problem is to compute the PV and PT equilibrium diagramsfor a single component fluid described by the van derWaals equation of state. Let us recall the key things we need to...
-
The following are the Ledger Balance (in thousands) extracted from the books of Vaishnavi Bank Ltd as on March 31, 2016. The bank's Profit and Loss Account for the year ended and Balance Sheet as at...
-
Canadian National Railway Company (CN) spans Canada and mid-America and provides freight transport services from the Atlantic Ocean to the Pacific Ocean and to the Gulf of Mexico. It is currently the...
-
Indicate whether each of the following statements is true or false by writing T or F i n t he a nswer c olumn. Pictures and words are legally obscene if they depict sexual conduct in a manner that is...
-
Fraud deterrence is centered on the fear of getting caught and the fear of getting punished. In your opinion, which is stronger and why?
-
Cisco's Sumptuous Burritos produces two burritos, chicken and steak, with the following characteristics: The total fixed costs for the company are $200,000. a. What is the anticipated level of...
-
A commercial building has 120 office spaces. Each office space has one, two, or three filing cabinets. There is one filing cabinet in 45 percent of the office spaces, and two filing cabinets in 35...
-
A compound has a molecular mass of 120 g/mol, and the information in the table below is the only other data available for a compound. Fill in all of the empty cells with your best estimate of the...
-
Suppose the ball in the previous problem is in contact with the wall for 1.1 ms. What average force does the wall exert on the ball?
-
If green light causes the ejection of electrons from a metal in a photoelectric effect experiment and yellow light does not, what would you expect to happen if red light were used to illuminate the...
-
Should Carla focus on her hit series or cede control and start the next new thing? explain.
-
Why is new jersey called the garden state and what do they grow?
-
What is the objective of the Control System Design?
-
https://academic.oup.com/jcr/article/36/3/478/1847025 I need a summary of this article i put above.
-
Why are rules used for scheduling rather than finding the optimal production schedule?
-
Abica Coffee Company roasts and packs coffee beans. The process begins by placing coffee beans into the Roasting Department. From the Roasting Department, coffee beans are then transferred to the...
-
Without solving, determine the character of the solutions of each equation in the complex number system. 3x 2 3x + 4 = 0
-
The helical gears in the simple geartrain have teeth numbers as labeled (Figure P8.17). The central gear rotates at 125 rpm and drives the two output shafts. Determine the speeds and rotation...
-
The spur gears in the compound geartrain have teeth numbers as labeled (Figure P8.18). (a) Determine the speed and rotation direction of the output shaft. (b) If the geartrain transfers 4 hp of...
-
The disk in a computers magnetic hard drive spins about its center spindle while the recording head C reads and writes data (Figure P8.19). The head is positioned above a specific data track by the...
-
Hill Corp. had 600,000 shares of common stock outstanding on January 1, issued 900,000 shares on July 1, and had income applicable to common stock of $1,050,000 for the year ending December 31, 2010....
-
Java owns 1000 shares of HAL Company common stock, which she acquired in 20X1 for $60,000. On January 15, 20X5, when the stock is worth $380 per share, Java received 1000 stock rights worth $37 per...
-
The community college instructor has asked for your help again. This time, he has asked for you to personally create a document he could give to his students. Write a 750- to 1,050-word paper in...

Study smarter with the SolutionInn App


