A gas has C P * = 35 J/mol K, and follows the equation of state:
Question:
A gas has CP* = 35 J/mol · K, and follows the equation of state: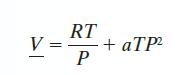 with a = 0.15 cm3/mol · bar2 · K. Find the change in A when the gas is compressed isothermally from T = 300 K and P = 1 bar to P = 10 bar.
with a = 0.15 cm3/mol · bar2 · K. Find the change in A when the gas is compressed isothermally from T = 300 K and P = 1 bar to P = 10 bar.
Transcribed Image Text:
V= RT P + aTP²
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
The change in Helmholtz free energy AA for an isotherm...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
An economy is initially described by the following equations C = 500 +0.50(Y-T) 1 = 500-25r d =Y - 25r G = 100 T = 500 M = 2000 P = 4 a. Write down the equations for the IS and LM curves and...
-
A gas has an ideal gas heat capacity of C P * = (7/2)R and is described by the equation of state: Z = 1 + (CP 2 )/(RT) with C = 100 cm 3 /bar mol. A . Find a general expression for the residual...
-
A gas with a flow rate of 300 mol/min enters a steady-state, adiabatic nozzle with negligible velocity at T = 500 K and P = 10 bar and leaves the nozzle at P = 1 bar. The gas has C P * = 40 J/mol K....
-
Name: 9. 11. Bell: Directions: Evaluate each expression using the order of operations. 1. 17-5-4+2 2. 40-32+8+5--2 Date: 5. 2018-(5+3)+7] 3.35-14+2+8 7.1+(-2-5)+(14-17)-4 25 +8+3 4+3 Unit 1: Algebra...
-
Assume you are a member of an international policy setting committee and are responsible for harmonizing audit report requirements internationally. Examine Exhibit 9-8. Based on the varying...
-
We would like to assess a service charge for cashing a check. The service charge depends on the amount of the check. If the check amount is less than $10, we will charge $1. If the amount is greater...
-
Figure P4.64 shows a fixed control volume. It has a volume \(V_{0}=1.0 \mathrm{ft}^{3}\), a flow area \(A=1.0 \mathrm{ft}^{2}\), and a length \(\ell_{0}=1.0 \mathrm{ft}\). Position \(x\) represents...
-
Tom, Jan, and Julie are IS majors at Great State University. These students have been assigned to a class project by one of their professors, requiring them to develop a new Web-based system to...
-
On January 1, 2021, Nash Corp. had 502,000 shares of common stock outstanding. During 2021, it had the following transactions that affected the Common Stock account. February 1 Issued 125,000 shares...
-
Imagine a compound has T C = 500 K and P C = 20 bar. Use the Peng-Robinson equation throughout this problem. A. Plot P- V at T = 400 K, T = 500 K, and T = 600 K, assuming the compound has = 0. B....
-
Steam is heated from an initial condition of saturated steam at P = 1.5 bar to a final state of P = 3 bar and T = 3008C. Use the steam tables to find the change in for this process.
-
Repeat Prob. 2/47, except now include the effects of aerodynamic drag. The drag force causes an acceleration component in ft/sec 2 of 0.005v 2 in the direction opposite the velocity vector, where v...
-
How do write Given the esteemed portfolio that you serve as the Hon. James Chuol Jiek Commissioner of Ayod County, Jonglei State and we are kindly reaching out to you to request a voluntary...
-
You are at a job interview and a perspective employer asks you to justify the value of accounting. In particular, they say it is ridiculous that they have to provide three different statements...
-
Agalinion company considering three alternative machines having following cost structure: MACHINE TYPE COST STRUCTURE A B C FIXED COST $400 $600 $900 VARIABLE COST $50 $40 $20 (1) Find the cross over...
-
hance's initial year with the venture is bad for business but provides an opportunity to offset his personal income from other sources. Nonetheless, the amount of loss Chance is entitled to is...
-
Debbie Debtor owed three debts to Carl Creditor, totaling $15,000. The first debt was $3,000, the second debt was $5,000, and the third debt was $7,000. Debbie needed to obtain additional gadgets...
-
What is an engagement letter, and what are its contents?
-
Find i 0 (t) for t > 0 in the circuit in Fig. 16.72 . 2 + Vo 1 7.5e-2t u(t) V ( +) 4.5[1 u(t)]V 0.5v. 1H
-
Use MATLAB to solve the following problem: x - 3y = 2 x + 5y = 18 4x - 6y = 10
-
a. Use MATLAB to find the coefficients of the quadratic polynomial y = ax 2 + bx + c that passes through the three points (x, y) = (1, 4), (4, 73), (5, 120). b. Use MATLAB to find the coefficients of...
-
Use the MATLAB program given in Table 8.52 to solve the following problems: a. Problem 3d d. b. Problem 11 Solve the following equations: c. Problem 14 Use MATLAB to solve the following problem: x -...
-
Prepare Sterling's journal entry on January 1 to record its purchase of stock investments with insignificant influence.
-
How to approach a discussion with a parent who is conflicted about immunizing a child? What are some barriers to effective communication in this scenario? What characteristics of effective...
-
Revise the sentences to emphasize the perspective of the audience and the "YOU" VIEW. A. We have prepared the enclosed form that may be used by victims to report identity theft to creditors. B. To...

Study smarter with the SolutionInn App


