Ammonia is synthesized in Figure 15-6 by the gas phase chemical reaction: N 2 + 3H 2
Question:
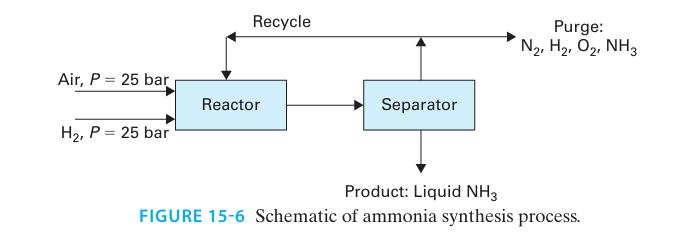
Ammonia is synthesized in Figure 15-6 by the gas phase chemical reaction:
N2 + 3H2 ↔ 2NH3
 The steady-state process is to be designed as follows.
The steady-state process is to be designed as follows.
∎ 30 mol/s of hydrogen gas and 15 mol/s of air, both compressed to 25 bar, enter the reactor.
∎ A recycle stream, also at 25 bar, enters the reactor.
∎ The stream leaving the reactor is at T = 300°C and P = 25 bar, and it is cooled to 0°C and P = 25 bar. This condenses much of the ammonia into liquid.
∎ The liquid and vapor are separated. The liquid ammonia is removed as product.
∎ 5% of the vapor stream is purged from the process, and the remainder is recycled. The purge and recycle streams have identical compositions.
Determine the molar flow rate of product, and the composition and flow rate of the purge stream, for each of the following cases.
A. The stream leaving the reactor is at equilibrium.
B. The stream leaving the reactor is not at equilibrium. Five percent of the hydrogen entering the reactor gets converted into ammonia.
Model air as 79 mol% nitrogen and 21 mol% oxygen, and assume the liquid product is pure ammonia (in other words, nitrogen, oxygen, and hydrogen are modeled as gases that are insoluble in ammonia)
Step by Step Answer:

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco





