Four vapor pressure data pointstwo representing solidvapor equilibrium and two representing liquidvapor equilibriumare available for a compound:
Question:
Four vapor pressure data points—two representing solid–vapor equilibrium and two representing liquid–vapor equilibrium—are available for a compound:
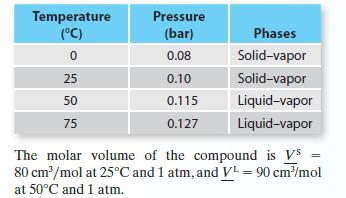
A. Give your best estimate of the triple point pressure and temperature for this compound.
B. Give your best estimate of pressure at which solid–liquid equilibrium occurs at T = 50°C.
C. Give your best estimate of the temperature at which liquid–vapor equilibrium occurs at P = 0.15 bar.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:





