One mole of a gas is placed in a closed system with a 20 L vessel initially
Question:
One mole of a gas is placed in a closed system with a 20 L vessel initially at T = 300 K. The vessel is then isothermally expanded to 40 L. The gas follows the equation of state:
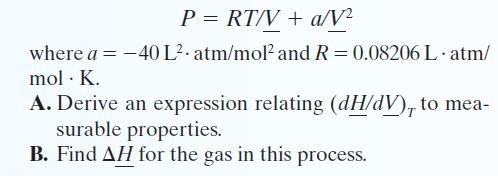
Transcribed Image Text:
P = RT/V + a/V² where a = -40 L² atm/mol² and R = 0.08206 L.atm/ mol. K. A. Derive an expression relating (dH/dV), to mea- surable properties. B. Find AH for the gas in this process.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
A Deriving an expression relating dHdV to measurable properties The enthalpy change dH for an isothe...View the full answer

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
Euler's original article about the Konigsberg Bridge Problem, which is dated 1736, presents a second similar problem with two islands, four rivers flowing around them, and 15 bridges connecting...
-
One mole of a certain gas is contained in a vessel of volume V = 0.250 1. At a temperature T1 = 300 K the gas pressure is Pl = 90 atm, and at a temperature T2 = 350 K the pressure is p2 = 110 atm....
-
One mole of a certain ideal gas is contained under a weight-less piston of a vertical cylinder at a temperature T. The space over the piston opens into the atmosphere. What work has to be performed...
-
A house girl added 20g of sodium chloride (NaCl) to 80g of water (atomic masses are Na=23amu, Cl=35.5amu). Calculate a)Percent(w/w) of NaCl b)Mole fraction of NaCl
-
What role does the attest function play in international financial statement analysis?
-
What is the output from the following? >>> a = 3 >>> b = -5 >>> x = a * b >>> print x
-
Assume the temperature of the exhaust in an exhaust pipe can be approximated by \(T=T_{0}\left(1+a e^{-b x} ight)[1+c \cos (\omega t)]\), where \(T_{0}=100^{\circ} \mathrm{C}, a=3, b=0.03...
-
Berkman Wholesalers accepts from Almonte Stores a $6,200, 4-month, 9% note dated May 31 in settlement of Almontes overdue account. The maturity date of the note is September 30. What entry does...
-
A block of mass =309 g is dragged with a string across a rough horizontal table. The string tension is =2.47 N, and it pulls upward at an angle of =46.0 with the horizontal. At one particular...
-
A gas has an ideal gas heat capacity of C P * = (7/2)R and is described by the equation of state: Z = 1 + (CP 2 )/(RT) with C = 100 cm 3 /bar mol. A . Find a general expression for the residual...
-
Demonstrate that if a gas follows the ideal gas law, (@U/P), and (@U/V), are equal to 0.
-
In most compounds the solid phase is denser than the liquid phase. Why isnt this true for water?
-
Batz Corporation has set a target profit of $12000 for the next period. How many specialty notebooks (units) does the company need to sell to a hive the target profit?
-
determine how you could check the numbers in the financial reports are correct for food and drinks?
-
What strategies can businesses employ to facilitate effective training and smooth adoption of computerized accounting systems by their staff? How can organizations balance the need for user-friendly...
-
describe how mision statement of non profit organizations is difrent from profit ones?please explain in your own wording.
-
Examine the real options approach in capital budgeting and its relevance in valuing flexibility in investment decisions. How does this technique capture the value of managerial choices in uncertain...
-
Why might an auditor be concerned if all of the companys management authority is centered in one or two individuals?
-
Explain how the graph of each function can be obtained from the graph of y = 1/x or y = 1/x 2 . Then graph f and give the (a) Domain (b) Range. Determine the largest open intervals of the domain over...
-
Van der Pols equation has been used to describe many oscillatory processes. It is Plot y(t) for 3 = 1 and 0 t 20, using the initial conditions y(0) = 5, y(0) = 0. (1 ?) + 3 0 y
-
The equation of motion for a pendulum whose base is accelerating horizontally with an acceleration a(t) is Suppose that g = 9.81 m/s 2 , L " 1 m, and (0) = 0. Plot ,(t) for 0 t 10 s for the...
-
Van der Pols equation is This equation is stiff for large values of the parameter 3. Compare the performance of ode45 and ode15s for this equation. Use 3 =1000 and 0 t 3000, with the initial...
-
When you are a job candidate taking part in a team interview: avoid making eye contact when answering questions. focus on the person who is the team leader regardless of which person asked a...
-
As a new department manager, you attend a company meeting for everyone to get to know you and so you can understand everyone's role in the department. As others explain their roles, how can you...
-
59.3% complete Question To minimize downtime and ensure the high availability of critical services, an organization seeks to evaluate the readiness and effectiveness of its backup systems under...
Dudleys Handbook Of Practical Gear Design And Manufacture 4th Edition - ISBN: 0367649020 - Free Book

Study smarter with the SolutionInn App


